1i10
From Proteopedia
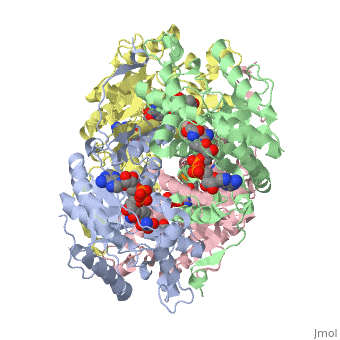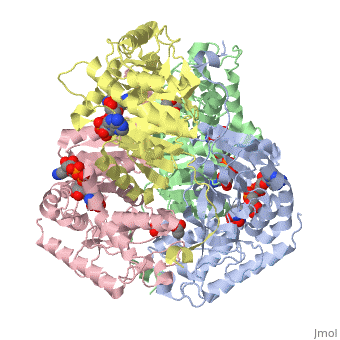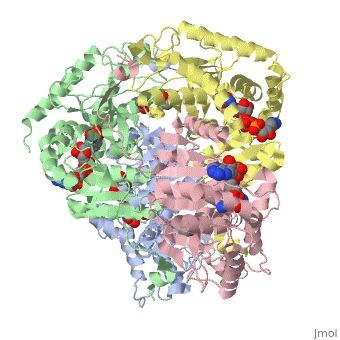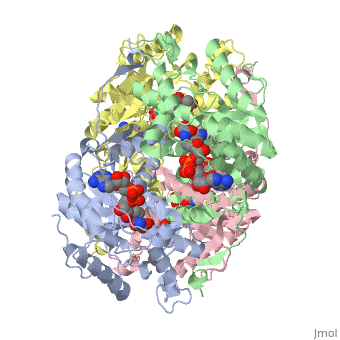
HUMAN MUSCLE L-LACTATE DEHYDROGENASE M CHAIN, TERNARY COMPLEX WITH NADH AND OXAMATE
Structural highlights
DiseaseLDHA_HUMAN Defects in LDHA are the cause of glycogen storage disease type 11 (GSD11) [MIM:612933. A metabolic disorder that results in exertional myoglobinuria, pain, cramps and easy fatigue.[1] FunctionEvolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedLactate dehydrogenase (LDH) interconverts pyruvate and lactate with concomitant interconversion of NADH and NAD(+). Although crystal structures of a variety of LDH have previously been described, a notable absence has been any of the three known human forms of this glycolytic enzyme. We have now determined the crystal structures of two isoforms of human LDH-the M form, predominantly found in muscle; and the H form, found mainly in cardiac muscle. Both structures have been crystallized as ternary complexes in the presence of the NADH cofactor and oxamate, a substrate-like inhibitor. Although each of these isoforms has different kinetic properties, the domain structure, subunit association, and active-site regions are indistinguishable between the two structures. The pK(a) that governs the K(M) for pyruvate for the two isozymes is found to differ by about 0.94 pH units, consistent with variation in pK(a) of the active-site histidine. The close similarity of these crystal structures suggests the distinctive activity of these enzyme isoforms is likely to result directly from variation of charged surface residues peripheral to the active site, a hypothesis supported by electrostatic calculations based on each structure. Proteins 2001;43:175-185. Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase.,Read JA, Winter VJ, Eszes CM, Sessions RB, Brady RL Proteins. 2001 May 1;43(2):175-85. PMID:11276087[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||||