Group:MUZIC
From Proteopedia

Contents |
The Z-disc of the muscle sarcomere
Introduction
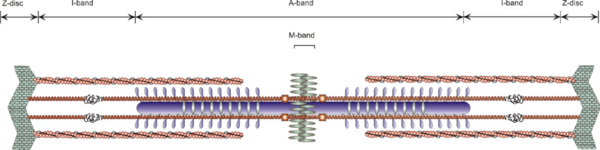
Although all types of muscle cells use actin and myosin for contraction, only in skeletal and cardiac muscle these proteins are organized into sarcomeric units. They are generally composed of ordered thick (myosin) and thin (actin, tropomyosin, troponin) filaments that slide past each other during contraction. The precise ultra-structural order of these filaments is of utmost importance for converting the molecular interactions produced by actin and myosin in each sarcomere into efficient contraction at the macroscopic level (Figure 1). In addition to those components responsible for active muscle operation, many other filamentous proteins, such as titin and nebulin, have important roles in myofibril structure formation and regulation. Titin, the largest known vertebrate gene product, connects the Z-discs to the central M-band [reviewed in [1]] and nebulin, which spans the length of the actin filaments [reviewed in [2][3][4]].
Figure 1 Schematic overview of the basic muscle contractile unit: sarcomere. Myosin filaments are shown in the middle in blue. Actin filaments are depicted as orange helical structures at the top and bottom of the diagram. Titin with its elastic properties in its I-band region is indicated by symbolic coiling (in grey). Nebulin is shown in grey, wrapping around actin filaments. The Z-disc and the M-band comprise additional protein networks, as shown by grey areas, crosslinking individual filament systems.
One of the functionally most complex sub-compartments of the sarcomere is the Z-disc that forms the lateral boundaries between adjacent sarcomeres. This region plays a central role as the site organizing thick filaments and titin into the molecular machinery that is required for muscle contraction and comprises the actin, titin, and nebulin filaments.
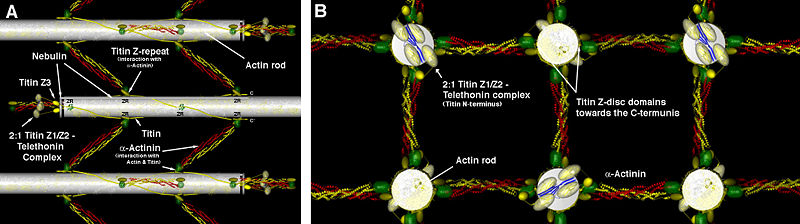
Figure 2 Z-disk ultrastructure. (A) Antiparallel arrangement of actin filaments (grey) interconnected by several layers of α-actinin (green and red) forming an overlapping zigzag like structure (B) The same view rotated by 90deg. Figure was prepared by Dr Stephan Lange (UCSD)
Thin filaments (actin) from adjacent sarcomeres are anchored at the Z-disc. In this area of the sarcomere, each actin filament overlaps with four filaments from the opposite sarcomere, forming a square lattice, which is cross-connected in a zig-zag pattern by α-actinin-2 [5] (Figure 2). This region plays a central role as the main anchoring point of the molecular machinery for muscle contraction comprising the actin, titin, and nebulin filaments [6][7].
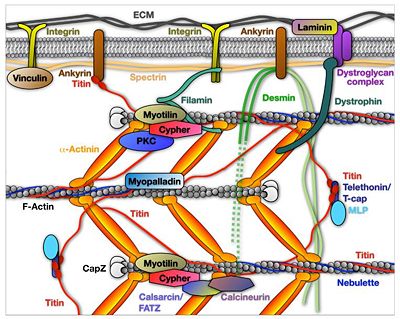
Z-discs are also implicated in mechanosensing and signaling to the nucleus, which contribute to maintenance of muscle homeostasis, and serve as attachment sites for desmin intermediate filaments and often for transverse tubules [reviewed in [8][9][10]]. The assembly of the Z-disc is controlled via N-terminal part of titin, which exhibits binding sites for α-actinin-2 as well as to additional Z-disc components. The most striking feature of muscle and Z-disc proteins, in particular, is the diversity of multiple protein-protein interactions that form part of a complex network, involving more than forty proteins[2][11][12] (Figure 3). For example, titin binds to α-actinin-2 via 45-residue sequence motifs, the so-called Z-repeats [13] and dimerizes at its N-terminal through the mediator protein telethonin [14].
Figure 3 Virtual molecular model of the sarcomeric Z-disc, integrating selected presently known protein components[15]. Opposing thin filaments and individual titin molecules (pink, horizontal) interdigitate at the Z-disc and are cross-linked by α-actinin dimers (green) www.e-heart.org
Links
- Interactome (Flash version) (static version)
- Protein Index (browse proteins annotated by MUZIC)
About MUZIC
The MUZIC network provides a unique mix of cellular and structural biology laboratories with a focus on muscle research and combines a series of complementary state-of-the art know-how and technologies ranging from high resolution (X-ray crystallography) and low resolution structural biology methods (SAXS, EM, cryo-EM tomography, atomic force microscopy) to a variety of cell biology oriented techniques, ranging from FRET and live-cell imaging, cellular and animal models to animal physiology. These are complemented by a biochemical and biophysical characterization of proteins and their complexes. about_muzic
References
- ↑ Lange S, Ehler E, Gautel M. From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol. 2006 Jan;16(1):11-8. Epub 2005 Dec 6. PMID:16337382 doi:10.1016/j.tcb.2005.11.007
- ↑ 2.0 2.1 Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637-706. Epub 2002 Apr 2. PMID:12142273 doi:10.1146/annurev.cellbio.18.012502.105840
- ↑ Ottenheijm CA, Granzier H, Labeit S. The sarcomeric protein nebulin: another multifunctional giant in charge of muscle strength optimization. Front Physiol. 2012;3:37. doi: 10.3389/fphys.2012.00037. Epub 2012 Feb 27. PMID:22375125 doi:10.3389/fphys.2012.00037
- ↑ Squire JM, Al-Khayat HA, Knupp C, Luther PK. Molecular architecture in muscle contractile assemblies. Adv Protein Chem. 2005;71:17-87. PMID:16230109 doi:10.1016/S0065-3233(04)71002-5
- ↑ Luther PK. The vertebrate muscle Z-disc: sarcomere anchor for structure and signalling. J Muscle Res Cell Motil. 2009;30(5-6):171-85. Epub 2009 Oct 15. PMID:19830582 doi:10.1007/s10974-009-9189-6
- ↑ Knoll R, Buyandelger B, Lab M. The sarcomeric Z-disc and Z-discopathies. J Biomed Biotechnol. 2011;2011:569628. doi: 10.1155/2011/569628. Epub 2011 Oct, 18. PMID:22028589 doi:10.1155/2011/569628
- ↑ Stromer MH. The cytoskeleton in skeletal, cardiac and smooth muscle cells. Histol Histopathol. 1998 Jan;13(1):283-91. PMID:9476658
- ↑ Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem. 2003 Apr 18;278(16):13591-4. Epub 2003 Jan 29. PMID:12556452 doi:10.1074/jbc.R200021200
- ↑ Gautel M. The sarcomeric cytoskeleton: who picks up the strain? Curr Opin Cell Biol. 2011 Feb;23(1):39-46. doi: 10.1016/j.ceb.2010.12.001. Epub, 2010 Dec 27. PMID:21190822 doi:10.1016/j.ceb.2010.12.001
- ↑ Voelkel T, Linke WA. Conformation-regulated mechanosensory control via titin domains in cardiac muscle. Pflugers Arch. 2011 Jul;462(1):143-54. doi: 10.1007/s00424-011-0938-1. Epub 2011 , Feb 25. PMID:21347754 doi:10.1007/s00424-011-0938-1
- ↑ Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med. 2006 Jun;84(6):446-68. Epub 2006 Jan 17. PMID:16416311 doi:10.1007/s00109-005-0033-1
- ↑ Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton. 2005 May;61(1):34-48. PMID:15810059 doi:10.1002/cm.20063
- ↑ Ohtsuka H, Yajima H, Maruyama K, Kimura S. Binding of the N-terminal 63 kDa portion of connectin/titin to alpha-actinin as revealed by the yeast two-hybrid system. FEBS Lett. 1997 Jan 13;401(1):65-7. PMID:9003807
- ↑ Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006 Jan 12;439(7073):229-33. PMID:16407954 doi:10.1038/nature04343
- ↑ Sheikh F, Bang ML, Lange S, Chen J. "Z"eroing in on the role of Cypher in striated muscle function, signaling, and human disease. Trends Cardiovasc Med. 2007 Nov;17(8):258-62. PMID:18021935 doi:10.1016/j.tcm.2007.09.002