Image:PEPC reaction mechanism.png
From Proteopedia
Size of this preview: 800 × 392 pixels
Full resolution (1024 × 502 pixel, file size: 48 KB, MIME type: image/png)
Summary
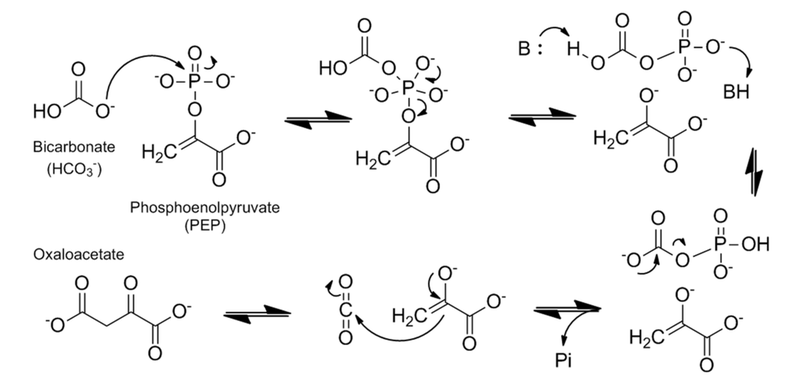
Catalytic mechanism of PEPC based on maize C4-PEPC and E. coli PEPC crystal structures and the three-step reaction model. The hydrophobic pocket is shown as yellow circles and the residues show maize numbering. Adapted from Izui K, Matsumura H, Furumoto T, Kai Y. Phosphoenolpyruvate carboxylase: a new era of structural biology. Annu Rev Plant Biol. 2004;55:69-84. PMID:15725057 doi:10.1146/annurev.arplant.55.031903.141619
Licensing
{{subst:Non-commercial from license selector}}
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 00:26, 30 June 2023 | Karsten Theis (Talk | contribs) | 1024×502 | 48 KB | From https://commons.wikimedia.org/wiki/File:PEP_Carboxylase_Mechanism.png, Tims2015, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons |
| 23:41, 25 June 2023 | Lucas Xavier da Cunha (Talk | contribs) | 611×400 | 160 KB | Catalytic mechanism of PEPC based on maize C4-PEPC and E. coli PEPC crystal structures and the three-step reaction model. The hydrophobic pocket is shown as yellow circles and the residues show maize numbering. Adapted from Izui K, Matsumura H, Furumoto T | |
| 22:42, 25 June 2023 | Lucas Xavier da Cunha (Talk | contribs) | 1078×706 | 397 KB | Catalytic mechanism of PEPC based on maize C4-PEPC and E. coli PEPC crystal structures and the three-step reaction model. The hydrophobic pocket is shown as yellow circles and the residues show maize numbering. Adapted from Izui K, Matsumura H, Furumoto |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
