NodS
From Proteopedia
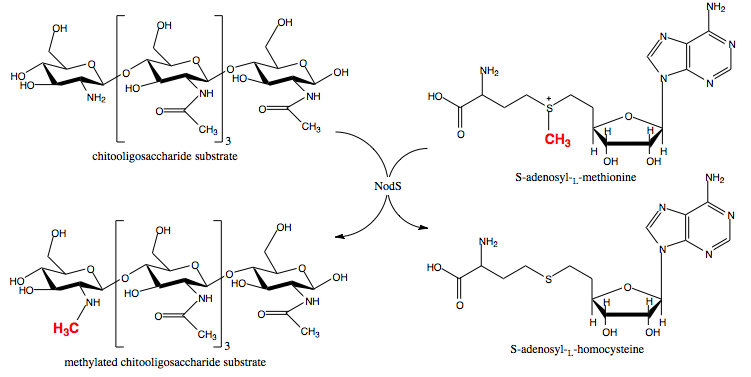
NodS is a bacterial methyltransferase involved in the pathway leading to the biosynthesis of Nod factor, an important signaling molecule released by rhizobial bacteria in the course of endosymbiotic interaction with legumes. NodS from Bradyrhizobium japonicum WM9 is the first S-adenosyl-L-methionine-dependent methyltransferase specific for chitooligosaccharide substrates, the structure of which has been solved. The structures with (3ofk) and without (3ofj) S-adenosyl-L-homocysteine ligand, which is chemically very similar to the methyl donor used by NodS, S-adenosyl-L-methionine, are available.[1][2]
Contents |
Biological significance
Rhizobia-legumes symbiosis
The endosymbiotic relationship between nitrogen-fixing rhizobial bacteria and certain plants (legumes) is of the utmost importance for the nitrogen cycle in the biosphere and hence an object of intense study by specialists from various areas of biology. What is more, although the use of artificial nitrogen-containing fertilizers made it possible to bypass the strict dependence on this process, its understanding is still crucial for the efficient agriculture. Biological nitrogen fixation requires nitrogenase, an enzyme present in, among others, rhizobial bacteria but absent in plants. The rhizobia are a diverse range of soil bacteria. It is thought that after the symbiotic capabilities were first acquired by some of them, they spread by means of horizontal transfer of a plasmid or genomic island containing genes important for the process. The symbiotic relationship between the two symbionts depends on the formation of invasion structures (nodules) by the host, allowing bacteria to enter the plant root, colonize a specific type of cells and be transformed into bacteroids which perform nitrogen fixation. As complementarity between various signals and their cognate receptors is required for the efficient symbiosis, its emergence most likely required coevolution of plants and bacteria.[3][4] One of the bacterial nodulating symbionts is Bradyrhizobium japonicum WM9, which is involved in a partnership with lupine and serradella legumes.[5]
Chemical signals
The very first stage of this process is an exchange of chemical signals between the symbionts. On sensing a flavonoid compound released by the plant, the bacteria produce a signalling molecule known as Nod factor, which is a lipochitooligosaccharide derivative. This molecule in turn induces nodule formation by the plant.[6][7][8] Many types of flavonoids and Nod factors are known and which ones are present influences the specificity of the interaction. It must be mentioned that while these two molecules are important signals and specificity determinants, there are numerous others.[9][10]
Nod factor synthesis
Nod factor synthesis involves the coordinated action of several rhizobial enzymes encoded by the nodulation-specific genes. NodC, NodB and NodA, found in nearly all rhizobial strains, synthesize the main part of Nod factor's structure, while several others, including NodS, NodU, NolO, NodL, and NolL, introduce species- and strain-specific modifications, such as N-methylation, carbamoylation, acetylation, fucosylation etc.[11][12]
Structure and function of NodS
| |||||||||||
3D structures of NodS
3ofj – BrNodS – Bradyrhibozium
3ofk – BrNodS + adenosyl homocysteine
2ocx, 2hhc, 2hlh – BrNodZ
1fh1 – NodF – Rhibozium leguminosarum
Reference
- ↑ Cakici O, Sikorski M, Stepkowski T, Bujacz G, Jaskolski M. Cloning, expression, purification, crystallization and preliminary X-ray analysis of NodS N-methyltransferase from Bradyrhizobium japonicum WM9. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Dec 1;64(Pt, 12):1149-52. Epub 2008 Nov 28. PMID:19052372 doi:10.1107/S174430910803604X
- ↑ Cakici O, Sikorski M, Stepkowski T, Bujacz G, Jaskolski M. Crystal Structures of NodS N-Methyltransferase from Bradyrhizobium japonicum in Ligand-Free Form and as SAH Complex. J Mol Biol. 2010 Oct 21. PMID:20970431 doi:10.1016/j.jmb.2010.10.016
- ↑ Martinez-Romero E. Coevolution in Rhizobium-legume symbiosis? DNA Cell Biol. 2009 Aug;28(8):361-70. PMID:19485766 doi:10.1089/dna.2009.0863
- ↑ Debelle F, Moulin L, Mangin B, Denarie J, Boivin C. Nod genes and Nod signals and the evolution of the Rhizobium legume symbiosis. Acta Biochim Pol. 2001;48(2):359-65. PMID:11732607
- ↑ Stepkowski T, Swiderska A, Miedzinska K, Czaplinska M, Swiderski M, Biesiadka J, Legocki AB. Low sequence similarity and gene content of symbiotic clusters of Bradyrhizobium sp. WM9 (Lupinus) indicate early divergence of "lupin" lineage in the genus Bradyrhizobium. Antonie Van Leeuwenhoek. 2003;84(2):115-24. PMID:14533715
- ↑ Oldroyd GE, Downie JA. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol. 2008;59:519-46. PMID:18444906 doi:10.1146/annurev.arplant.59.032607.092839
- ↑ Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol. 2007 Aug;5(8):619-33. PMID:17632573 doi:10.1038/nrmicro1705
- ↑ Denarie J, Debelle F, Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497-531. PMID:1444265 doi:http://dx.doi.org/10.1146/annurev.mi.46.100192.002433
- ↑ Downie JA. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol Rev. 2010 Mar;34(2):150-70. Epub 2009 Dec 15. PMID:20070373 doi:10.1111/j.1574-6976.2009.00205.x
- ↑ Fauvart M, Michiels J. Rhizobial secreted proteins as determinants of host specificity in the rhizobium-legume symbiosis. FEMS Microbiol Lett. 2008 Aug;285(1):1-9. PMID:18616593 doi:10.1111/j.1574-6968.2008.01254.x
- ↑ D'Haeze W, Holsters M. Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology. 2002 Jun;12(6):79R-105R. PMID:12107077
- ↑ Riely BK, Ane JM, Penmetsa RV, Cook DR. Genetic and genomic analysis in model legumes bring Nod-factor signaling to center stage. Curr Opin Plant Biol. 2004 Aug;7(4):408-13. PMID:15231263 doi:10.1016/j.pbi.2004.04.005
- ↑ Geelen D, Leyman B, Mergaert P, Klarskov K, Van Montagu M, Geremia R, Holsters M. NodS is an S-adenosyl-L-methionine-dependent methyltransferase that methylates chitooligosaccharides deacetylated at the non-reducing end. Mol Microbiol. 1995 Jul;17(2):387-97. PMID:7494487
- ↑ Schubert HL, Blumenthal RM, Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem Sci. 2003 Jun;28(6):329-35. PMID:12826405
- ↑ Rao ST, Rossmann MG. Comparison of super-secondary structures in proteins. J Mol Biol. 1973 May 15;76(2):241-56. PMID:4737475
- ↑ Cakici O, Sikorski M, Stepkowski T, Bujacz G, Jaskolski M. Crystal Structures of NodS N-Methyltransferase from Bradyrhizobium japonicum in Ligand-Free Form and as SAH Complex. J Mol Biol. 2010 Oct 21. PMID:20970431 doi:10.1016/j.jmb.2010.10.016
Proteopedia Page Contributors and Editors (what is this?)
Marcin Jozef Suskiewicz, Alexander Berchansky, David Canner, Michal Harel