Rotation of 4 degrees of FIX
From Proteopedia
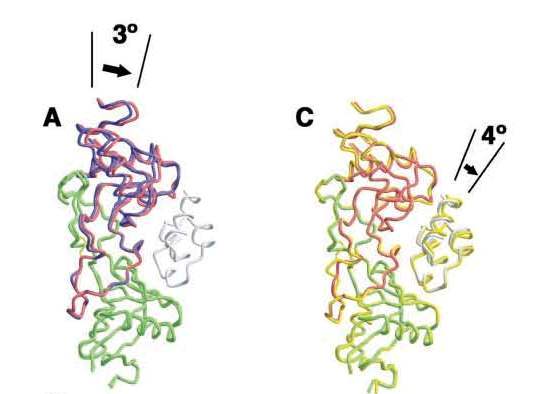
Movement of A chain of IX-bp caused by induced fitting and of IXGD1–46 caused by Mg2+ ions [1]. A, interaction patch for IX-bp (residues 100–110 of IX-bp B chain) is overlaid. Complexed and uncomplexed IX-bp A and B chains are shown in red and green for A and in blue and green for B, respectively. B, hydrophobic and hydrophilic interactions involving Lys22 of IXGD1–46 and Thr62, Lys63, and Glu108 of IX-bp A chain resulting from the movement of the A chain. The surface model was calculated using GRASP (41). This interaction was not observed, formed under Mg2+-free condition. C, Mg2+-bound IXGD1–46/IX-bp complex is shown in red and green, and the Mg2+-free (Ca2+ only) complex is depicted by yellow ribbon representation. Both structures are superimposed as a result of the position of C atoms of IX-bp.
Additional Resources
For additional information, see: Hemophilia
References
- ↑ Shikamoto Y, Morita T, Fujimoto Z, Mizuno H. Crystal structure of Mg2+- and Ca2+-bound Gla domain of factor IX complexed with binding protein. J Biol Chem. 2003 Jun 27;278(26):24090-4. Epub 2003 Apr 14. PMID:12695512 doi:10.1074/jbc.M300650200