Srp20-Human Alternative Splicing Factor
From Proteopedia
Contents |
Overview
The SRp20 protein is an alternative splicing factor for several genes found in homo sapiens as well as many other eukaryotes.Introduction
History
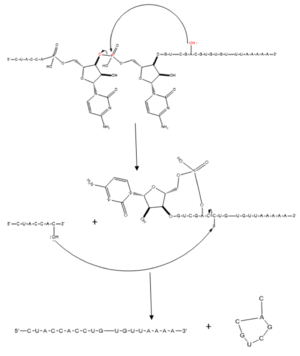
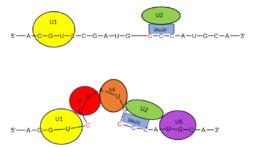
Splicing is one step in the process of RNA maturation that cuts out introns and joins exons together. Both the spliceosome, a complex of snRNAs (U1, U2, etc.), and splicing factors like SRp20 interact with intron consensus sequences in the pre-mRNA to regulate this process. Alternative splicing allows one mRNA molecule to produce numerous proteins that perform different functions in a cell by inclusion and exclusion of RNA sequences. There are two main families of splicing factors: Serine-Arginine rich (SR) proteins and heterogeneous nuclear RiboNucleoProteins (hnRNPs). The SRp20 protein belongs to the SR protein family. All SR proteins are defined by a RNA-binding domain at the N-terminus and a serine-arginine rich domain at the C-terminus[2]. The discovery of this family started in the 1900s with the SF2 (SRp30a) protein and has since come to include twelve proteins, all of which act as splicing factors. SRp20 was first discovered in calf thymus when it was separated with several other SR proteins based on their molecular weight[2]. The SRp20 protein, called X16 originally, was actually discovered first in an earlier paper studying different genes that change expression during B lymphocyte development[1]. At the time, the protein was assumed to play a role in RNA processing and cellular proliferation, a finding that was later proved in further studies[3][4]. The SRp20 protein has been shown to play a role in cancer progression and neurological disorders, specifically through alternative splicing. For example, SRp20 has been shown to play a role in alternative splicing of the Tau protein, an integral protein in the progression of Alzheimer’s disease[1]. SRp20 has even been found to serve as a splicing factor for its own mRNA, influencing the inclusion of exon 4[1]. Another function of SRp20 is its role in export of mRNA out of the nucleus, notably H2A histone mRNA export[5].
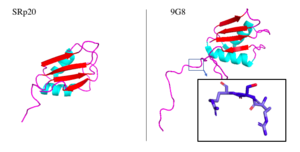
Structure and Function
| |||||||||||
Medical Significance
Cancer
SRp20 has been linked to cancer in many instances, as have other alternative splicing proteins. SRp20 has been seen to activate the alternative splicing of the CD44 adhesion molecule by facilitating the splicing of exon v9, which is important for CD44 functionality. Loss of function of SRp20 leading to loss of function of CD44 will lead to loss of “stickiness” of cells, allowing cancer cells to spread to other areas of the body. SRp20 can also affect the alternative splicing of oncogenes and tumor suppressors. Expression of the signaling pathway for SRp20 translation is directly triggered by oncogenic signaling pathways. For example, the Wnt pathway, that is associated with cancer development, also drives further expression of SRp20 and other SR proteins[8]. These proteins aid in cell growth and proliferation, by increasing expression of proteins that are involved in splicing. One study found that silencing SRp20 slowed cell proliferation, further supporting the idea that SRp20 aids in the process[1]. Cell cycle regulator proteins FoxM1, Cdc25B, and PLK1 regulate the G2/M cycles and are alternatively spliced by SRp20. These proteins are also associated with regulation of cell apoptosis. When these genes are overexpressed due to overexpressed SRp20, cells will no longer be able to apoptose when they are damaged, and the cell cycle will continue without regulation, causing overactive cell proliferation and eventually cancer. SRp20 has been seen to be overexpressed in ovarian, lung, breast, stomach, skin, bladder, colon, liver, thyroid, and kidney cancer tissue[9]. SRp20 also regulates genes associated with cellular senescence, or cellular immortality, which contributes to cancer formation. Specifically, SRp20 alternatively splices the TP53 gene, which generates the p53 senescence protein, promoting "cell immortality," which is characteristic of cancer.
Neurological Disorders
Along with cancer, SRp20 mutations have been linked to Alzheimers, a neurodegenerative disorder. SRp20 is involved in alternative splicing of a wide array of RNAs, including that of the TRKB gene which generates TrkB-Shc transcripts that are involved in generation of the disorder. SRp20 also promotes exclusion of exon 10 in the TAU gene, a gene important in establishing microtubules in axons or transport processes. Underexpression of SRp20 results in dysfunction of the TAU gene, less microtubule functionality, and the brain deterioration characteristic of Alzheimers[10][8].
Other Genetic Disorders
SRp20 and other SR proteins have been shown to prevent R-loops from forming, triple-stranded nucleic acid structures consisting of RNA and DNA. R-loops have been known to promote mutations, recombination, and chromosome rearrangement. One proposed mechanism for R-loop prevention by SRp20 is that SRp20, being a protein involved in RNA metabolism, is a binding partner of the TOP1 protein. Underexpression of TOP1 also promotes R-loop formation. TOP1 has kinase activity that potentially phosphorylates the SR domain of SRp20, which contributes to its function. Underexpression of TOP1 would lead to loss-of-function of SRp20, which could lead to cancer and Alzheimers as mentioned above, as well as cause R-loops to form. R-loops have been associated with disorders such as Down Syndrome[11].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Corbo C, Orru S, Salvatore F. SRp20: an overview of its role in human diseases. Biochem Biophys Res Commun. 2013 Jun 21;436(1):1-5. doi:, 10.1016/j.bbrc.2013.05.027. Epub 2013 May 16. PMID:23685143 doi:http://dx.doi.org/10.1016/j.bbrc.2013.05.027
- ↑ 2.0 2.1 Zahler AM, Lane WS, Stolk JA, Roth MB. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992 May;6(5):837-47. PMID:1577277
- ↑ Ayane M, Preuss U, Kohler G, Nielsen PJ. A differentially expressed murine RNA encoding a protein with similarities to two types of nucleic acid binding motifs. Nucleic Acids Res. 1991 Mar 25;19(6):1273-8. PMID:2030943
- ↑ Caceres JF, Kornblihtt AR. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 2002 Apr;18(4):186-93. PMID:11932019
- ↑ 5.0 5.1 5.2 5.3 5.4 Hargous Y, Hautbergue GM, Tintaru AM, Skrisovska L, Golovanov AP, Stevenin J, Lian LY, Wilson SA, Allain FH. Molecular basis of RNA recognition and TAP binding by the SR proteins SRp20 and 9G8. EMBO J. 2006 Nov 1;25(21):5126-37. Epub 2006 Oct 12. PMID:17036044
- ↑ 6.0 6.1 Shepard PJ, Hertel KJ. The SR protein family. Genome Biol. 2009;10(10):242. doi: 10.1186/gb-2009-10-10-242. Epub 2009 Oct 27. PMID:19857271 doi:http://dx.doi.org/10.1186/gb-2009-10-10-242
- ↑ 7.0 7.1 Huang Y, Gattoni R, Stevenin J, Steitz JA. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell. 2003 Mar;11(3):837-43. PMID:12667464
- ↑ 8.0 8.1 Corbo C, Orru S, Gemei M, Noto RD, Mirabelli P, Imperlini E, Ruoppolo M, Vecchio LD, Salvatore F. Protein cross-talk in CD133+ colon cancer cells indicates activation of the Wnt pathway and upregulation of SRp20 that is potentially involved in tumorigenicity. Proteomics. 2012 Jun;12(12):2045-59. doi: 10.1002/pmic.201100370. PMID:22623141 doi:http://dx.doi.org/10.1002/pmic.201100370
- ↑ Jia R, Li C, McCoy JP, Deng CX, Zheng ZM. SRp20 is a proto-oncogene critical for cell proliferation and tumor induction and maintenance. Int J Biol Sci. 2010 Dec 15;6(7):806-26. PMID:21179588
- ↑ Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998 Nov 2;143(3):777-94. PMID:9813097
- ↑ Naro C, Bielli P, Pagliarini V, Sette C. The interplay between DNA damage response and RNA processing: the unexpected role of splicing factors as gatekeepers of genome stability. Front Genet. 2015 Apr 15;6:142. doi: 10.3389/fgene.2015.00142. eCollection 2015. PMID:25926848 doi:http://dx.doi.org/10.3389/fgene.2015.00142