Student Project 4 for UMass Chemistry 423 Spring 2015
From Proteopedia
Contents |
Dengue virus methyltransferase bound to a SAM-based inhibitor-3P8Z
by Michael Bresnahan, Matthew Caissy, Jonathan Dullea, James Eyke, Adlina Hasni, Xuanting Wang
Student Projects for UMass Chemistry 423 Spring 2015
Introduction
Dengue virus, from the genus Flativirus, is a type of virus that causes yellow fever, dengue fever, tick-borne encephalitis, and Japanese B. encephalitis.[1]
|
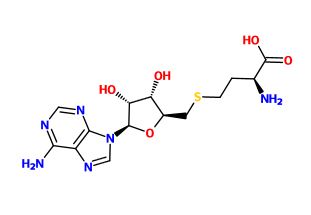
is developed as an antiviral drug to target dengue virus infection by modulating flavivirus MTase. Dengue virus MTase is essential for viral replication because it has been found to catalyze two distinct methylation reactions at the guanine N7 and ribose 2’O position of the viral RNA cap structure. S-adenosyl-L-methionine (SAM) on the viral enzyme acts as a methyl group donor during methylation reaction due to its highly favorable reaction energetic.[2] Dengue virus MTase bound to a SAM-based inhibitor is able to control the viral infection of dengue virus by preventing SAM from methylating and limiting the viral replication. This enzyme has a total of two chains that are represented in a 1-sequence-unique entity. It has two different types of ligand which are . SAH known to be an inhibitor of the methylation reaction of the virus. Chemical derivatizations of SAH have been shown to have increased selective activity towards Dengue Virus Methyltransferase, while demonstrating no activity toward human enzymes. This reduces harmful or toxic activity in the human body.Since ligands 36A and SAH have similar chemical structure, it is predicted that ligands 36A and SAH each limits methylation at different position of the viral RNA. [3]
Overall Structure
|
Dengue Virus Methyltransferase bound to a SAM-Based inhibitor is a dimer, with two egg-like halves pointing at each other via their smaller point. The protein is composed of fourteen alpha helices, seven in each half of the dimer. There are eighteen beta sheets, nine in each half, in the protein. illustrated below shows the charged groups in maroon and the hydrophobic groups are shown in lime green. The alpha helices are towards the outside of each half of the protein dimer and are both polar and non-polar. The beta sheets are closer to the insides of the two halves and are mostly non-polar. Almost all of the polar amino acids are on the outside of the protein, where the non-polar groups are both on the inside and outside of the protein.
Binding Interactions
|
This viral protein functions to methylate the viral RNA cap of originating from the dengue virus. In order to do this, the protein binds a S-adenosyl-l-methionine ligand (SAM). The SAM ligand acts as the methyl group donor. It must also bind the 5' end of the RNA cap. MTases are highly conserved, thus blocking only the desired viral protein can prove to be difficult.
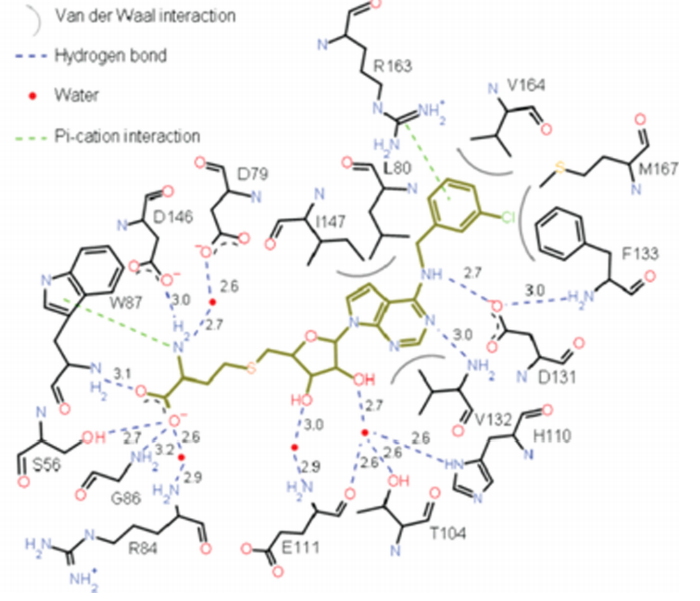
In order to block this binding a similar ligand to the SAM ligand was employed. The ligand chosen as an inhibitor is the S-Adenosyl-L-homocysteine ligand. This ligand is similar to the SAM ligand with the one exception of a hydrogen where the methyl group donor is in the native substrate. The protein without the inhibitor bound is in conformation.[4]. [5]
does not change the structure of the protein to a great degree, the RMS difference of the Cα atoms is 0.49 angstroms.
The relatively . Favorable hydrogen bonds are satisfied in this interaction along with hydrophobic interactions between the non-polar residues and the non-polar portions of the inhibitor. The bonds are satisfied with the following amino acids on the protein, Phe-133, Ile-147, Gly-148, Glu-149, Arg-160, Arg-163, Val-164, and Leu-182. These residues help to stabilize the charges present on the ligand; they are not all strictly hydrophilic, however they do have portions that allow for hydrogen bonding.[6]
Competitive binding on this active site could prove to be an effective drug target; if the SAM ligand cannot bind then the viral RNA cap will not be methylated. This could lead to a less viable virus due to a incorrectly formed 5' cap on the viral RNA.
Additional Features
|
structure shows semblance to other members of the Flavivirus family as can be seen when comparing it to , and . Structural similarity especially appears within the binding pocket of these MTases regardless of SAH/SAM ligands and therefore the methylated-state of the SAM molecule shows unimportant affect over ligand-protein reciprocity.
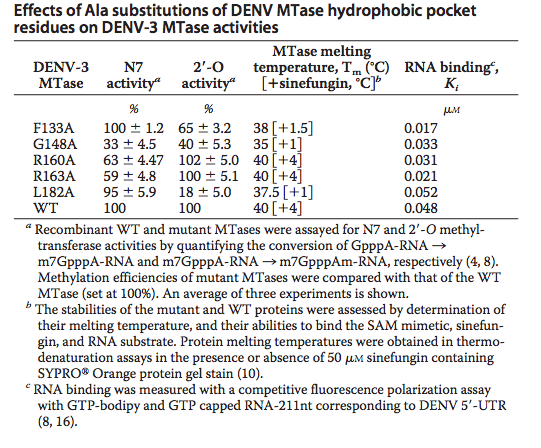
A unique hydrophobic pocket (viewable in and ) was identified in this protein above the adenine base (of SAH) and was first identified in West Nile Virus Methyltransferase. It can be found within conserved amino acids Phe-133, Ile-147, Gly-148, Glu-149, Arg-160, Arg 163, Val-164, and Leu-182. Phe-133 and Ile-147 are of particular interest because of their functionality in the binding cavity and their formation of the hydrophobic pocket. Alanine mutations revealed various effects on the production and viral titer of DENV-3 MTase. The Table below[7], shows the various effects of Alanine mutations at selected sites. These transfectation experiments revealed that mutations at the N-7 (R160A, R160A) or 2’-O (F133A, L18A) methylation activities were significantly depreciated. Melting temperatures in the table indicate low mis-folding in the mutant MTases with respect to the wild type. These attributes confirm observations in WNV MTase; that the identified hydrophobic pocket is critical to Flavivirus cap methylations and replication within cell environments. Diminishment in these properties decrease the virus’s overall potency. Therefore selective inhibition of DENV-3 MTase at this unique binding cavity becomes a viable antiviral strategy.[8]
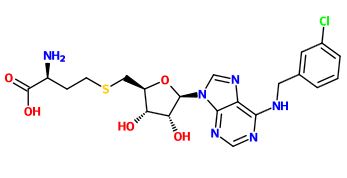
Compounds were experimentally used to test for inhibitory strength and several key conclusions were made. SAH is non-selective and therefore inhibits eukaryotic, bacterial and viral DENV, WNV, human RNA, DNA, and histone MTases. Attachment of a benzyl ring increased selectivity for DENV MTase, however extending the aryl group by a length of one carbon reduced inhibition significantly due to hydrophobic steric hindrance, and increased substituent flexibility causing bond attenuation. Meta-substitution on the benzyl moiety caused significant increase in inhibition compared to SAH. Larger bromine and iodine groups, however, showed reduced inhibition suggesting steric hindrance from bulky groups reduces inhibition. The N6-substituted benzy ring with chloro functionality revealed rotation of Phe-133 to accommodate the bulky benzyl group, and strong cation-pi interaction between Arg-163 and the aryl-ring, the overall effect of which was tighter binding and subsequent better inhibition.[9]
Quiz Question 1
Question1:
|
Why are ligands on Dengue Virus Methyltransferase predicted to have similar function of inhibiting Dengue Virus MTase?
A). They are both nonpolar ligands.
B). They are very similar in structure.
C). They are both located on the outside of the enzyme.
D). None of the above.
Quiz Question 2
Question 2: Compound 36A in question 1 showed improved inhibitory potency against DENV MTase activity with respect to SAH. Utilizing the diagram below, what would be the best explanation for such an improvement? [10]
A.) Improved Hydrogen bonding
B.) Steric stability within the hydrophobic pocket
C.) Cation-pi interaction with N-6 aryl ring and R163
D.) Extension of carbon chain from the N-6 to the aryl substituent increasing flexibility and thus creating strong a stronger binding affinity for the hydrophobic pocket
E.) None of the Above
See Also
- http://www.uniprot.org/uniprot/C1KBQ3
- http://www.ebi.ac.uk/pdbsum/3p8z
- http://proteopedia.org/wiki/index.php/3p8z
- http://www.proteopedia.org/wiki/index.php/3p97
- http://www.proteopedia.org/wiki/index.php/SAM-dependent_methyltransferase
Credits
Introduction - Adlina Hasni, Xuanting Wang
Overall Structure - Michael Bresnahan
Drug Binding Site - Jon Dullea
Additional Features - Matthew Caissy
Quiz Question 1 - Xuanting Wang
Quiz Question 2 - Matthew Caissy
References
- ↑ AnonymousFlavivirus. http://www.britannica.com/EBchecked/topic/209809/flavivirus (accessed April 2, 2015).
- ↑ Schmidt, T.; Schwede, T.; Meuwly, M. Computational Analysis of Methyl Transfer Reactions in Dengue Virus Methyltransferase. J Phys Chem B 2014, 118, 5882-5890. DOI: 10.1021/jp5028564
- ↑ Lim SP, Sonntag LS, Noble C, Nilar SH, Ng RH, Zou G, Monaghan P, Chung KY, Dong H, Liu B, Bodenreider C, Lee G, Ding M, Chan WL, Wang G, Jian YL, Chao AT, Lescar J, Yin Z, Vedananda TR, Keller TH, Shi PY. Small molecule inhibitors that selectively block dengue virus methyltransferase. J Biol Chem. 2011 Feb 25;286(8):6233-40. Epub 2010 Dec 8. PMID:21147775 doi:10.1074/jbc.M110.179184
- ↑ http://proteopedia.org/wiki/index.php/4r8r
- ↑ http://ac.els-cdn.com/S0166354214002563/1-s2.0-S0166354214002563-main.pdf?_tid=26b60158-c357-11e4-9d55-00000aab0f6b&acdnat=1425574160_e6994207ddfee772f88490227e693ef4
- ↑ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057852/
- ↑ J Biol Chem. 2011 Feb 25;286(8):6233-40. Epub 2010 Dec 8. PMID:21147775 doi:10.1074/jbc.M110.179184
- ↑ J Biol Chem. 2011 Feb 25;286(8):6233-40. Epub 2010 Dec 8. PMID:21147775 doi:10.1074/jbc.M110.179184
- ↑ J Biol Chem. 2011 Feb 25;286(8):6233-40. Epub 2010 Dec 8. PMID:21147775 doi:10.1074/jbc.M110.179184
- ↑ image taken from. J Biol Chem. 2011 Feb 25;286(8):6233-40. Epub 2010 Dec 8. PMID:21147775 doi:10.1074/jbc.M110.179184