Translocation domain
From Proteopedia
The Colicin translocation domain is found at the T terminus of the colicin protein. This structure is involved in the recruitment of a group of proteins, either from the Tol system or the Ton system, after the receptor binding domain of the colicin has bound to an outer membrane receptor of the target cell, such as BtuB. The proteins recruited by the T domain are then responsible in some way for the translocation of the colicin into the cytoplasm of the target cell, through a mechanism as yet unidentified, although a few hypotheses about how this occurs do exist.
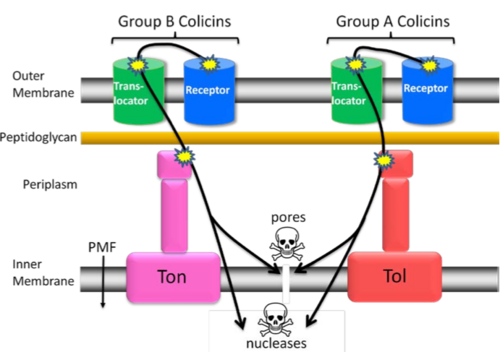
Colicins that recruit the proteins from the Tol system are classed as being group A colicins, and those using the Ton system are group B colicins. Group A colicins also recruit a co-receptor to aid their translocation, such as OmpF or Tol C[2], which is not something also seen in Group B colicins[3]. They may indiscriminately recruit other membrane proteins to aid translocation, that have transient interaction - this would explain why they have not yet been identified. The two systems, tol and ton, import the proteins through two distinct mechanisms.
Once they have bound to the receptor and recruited translocation proteins, many colicins are known to unfold before moving across the membrane into the cytoplasm. However, the diameter of the unfolded peptide exceeds the diameter of the pores formed by the proteins recruited into the area[4][5]. In both A and B colicins, it is known that the central receptor binding domain does not cross into the cell, but stays bound to the receptor at the cell surface[6].
Group A colicins
All except for Colicin N require two outer membrane proteins, both a receptor, such as BtuB, and a co-receptor, such as OmpF or TolC. One proposed model for the translocation across the outer membrane is this[7]:
The outer membrane receptor binds to the colicin, and the co-receptor is recruited and interacts with the T domain of the colicin[8]. The T domain passes through the pore formed by the co-receptor, a β barrel region, and recruits the Tol proteins[9]. The interaction with the tol proteins may disrupt the outer membrane to allow translocation of the C domain[10]The C domain of the colicin enters the periplasm, probably through a separate co-receptor[11].
The T domain then directs transit across the periplasm of the C domain, through interactions with the Tol proteins with specific binding sequences. The C domain is what is required of the colicin for the killing of the cell, and is sufficient to kill the cell without the other domains once it is inside.
Group B colicins
All except for Colicin 5 and Colicin 10 use a single outer membrane protein for both binding and transport of the T domain into the periplasm, such as the Cir protein. A proposed translocation mechanism of the colicin is[12]:
Colicin binding to the outer membrane protein promotes an interaction between the outer membrane protein, and TonB in the periplasm. Energy from the PMF may open a pore in the outer membrane protein by removal of the plug domain of the protein, and allowing the T domain of the colicin to enter into the periplasm. Once inside, the T domain and TonB interact to allow entry of the cytotoxic domain.
Crucial for the interaction between the colicin and TonB is the TonB box, a conserved sequence found in the T domain of the colicin. More than one may be present. Further interactions between these direct transit across the periplasm of the C domain
References
- ↑ Kleanthous C. Swimming against the tide: progress and challenges in our understanding of colicin translocation. Nat Rev Microbiol. 2010 Dec;8(12):843-8. Epub 2010 Nov 9. PMID:21060316 doi:10.1038/nrmicro2454
- ↑ Mock M, Pugsley AP. The BtuB group col plasmids and homology between the colicins they encode. J Bacteriol. 1982 Jun;150(3):1069-76. PMID:6281233
- ↑ Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D. Colicin biology. Microbiol Mol Biol Rev. 2007 Mar;71(1):158-229. PMID:17347522 doi:10.1128/MMBR.00036-06
- ↑ Benedetti H, Lloubes R, Lazdunski C, Letellier L. Colicin A unfolds during its translocation in Escherichia coli cells and spans the whole cell envelope when its pore has formed. EMBO J. 1992 Feb;11(2):441-7. PMID:1537329
- ↑ Cowan SW, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit RA, Jansonius JN, Rosenbusch JP. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727-33. PMID:1380671 doi:http://dx.doi.org/10.1038/358727a0
- ↑ Benedetti H, Lloubes R, Lazdunski C, Letellier L. Colicin A unfolds during its translocation in Escherichia coli cells and spans the whole cell envelope when its pore has formed. EMBO J. 1992 Feb;11(2):441-7. PMID:1537329
- ↑ Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D. Colicin biology. Microbiol Mol Biol Rev. 2007 Mar;71(1):158-229. PMID:17347522 doi:10.1128/MMBR.00036-06
- ↑ Housden NG, Loftus SR, Moore GR, James R, Kleanthous C. Cell entry mechanism of enzymatic bacterial colicins: porin recruitment and the thermodynamics of receptor binding. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13849-54. Epub 2005 Sep 15. PMID:16166265 doi:10.1073/pnas.0503567102
- ↑ Loftus SR, Walker D, Mate MJ, Bonsor DA, James R, Moore GR, Kleanthous C. Competitive recruitment of the periplasmic translocation portal TolB by a natively disordered domain of colicin E9. Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12353-8. Epub 2006 Aug 7. PMID:16894158
- ↑ Deprez C, Blanchard L, Guerlesquin F, Gavioli M, Simorre JP, Lazdunski C, Marion D, Lloubes R. Macromolecular import into Escherichia coli: the TolA C-terminal domain changes conformation when interacting with the colicin A toxin. Biochemistry. 2002 Feb 26;41(8):2589-98. PMID:11851406
- ↑ Zakharov SD, Zhalnina MV, Sharma O, Cramer WA. The colicin E3 outer membrane translocon: immunity protein release allows interaction of the cytotoxic domain with OmpF porin. Biochemistry. 2006 Aug 29;45(34):10199-207. PMID:16922495 doi:10.1021/bi060694+
- ↑ Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D. Colicin biology. Microbiol Mol Biol Rev. 2007 Mar;71(1):158-229. PMID:17347522 doi:10.1128/MMBR.00036-06
