User:Dannielle Ryman/Sandbox 2
From Proteopedia
|
Contents |
Background
Collagen is a structural protein found in abundance in connective tissues along with the breast tumor microenvironment. Collagen I specifically is the most abundant extracellular matix (ECM) protein found in the primary breast tumor, whereas other collagen types such as Collagen IV and III are commonly found in the basement membrane of the tumor microenvironment. During tumor development TACS (tumor associated collagen signaling) occurs. TACS is the process by which the ECM collagen reorganizes and forms complex fibrous bundles facilitating individual and aggregated primary cells to extravasate the primary tumor site and translocate to a secondary site.
Structure
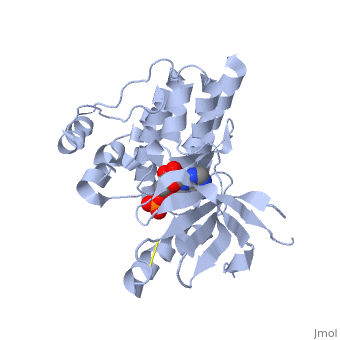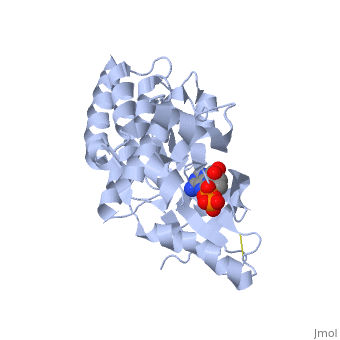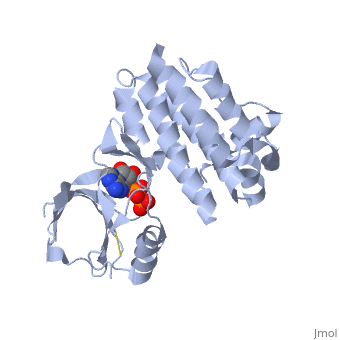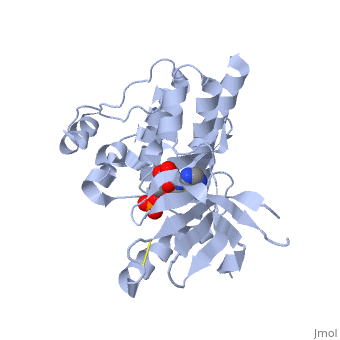
Focal adhesion kinase (FAK) activation by phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2)-induced conformational change. In the inactive state, FAK adopts a closed, auto-inhibited conformation through interactions between its four-point-one, ezrin, radixin, moesin (FERM) and kinase domains. Focal adhesion kinase (FAK) contains a FERM (protein 4.1, ezrin, radixin and moesin homology) domain, a kinase domain and a focal adhesion targeting (FAT) domain. The FAT domain recruits FAK to focal contacts by associating with integrin-associated proteins such as talin and paxillin. It also links FAK to the activation of Rho GTPases by binding to guanine nucleotide-exchange factors (GEFs) such as p190 RhoGEF. FAK contains three proline-rich regions (PRR1–3), which bind Src-homology-3 (SH3) domain-containing proteins such as p130Cas, the GTPase regulator associated with FAK (GRAF) and the Arf-GTPase-activating protein ASAP1. FAK is phosphorylated (P) on several tyrosine residues, including Tyr397, 407, 576, 577, 861 and 925. Tyrosine phosphorylation on Tyr397 creates a Src-homology-2 (SH2) binding site for Src, phospholipase Cgamma (PLCgamma), suppressor of cytokine signalling (SOCS), growth-factor-receptor-bound protein 7 (GRB7), the Shc adaptor protein, p120 RasGAP and the p85 subunit of phosphatidylinositol 3-kinase (PI3K). Phosphorylation of Tyr576 and Tyr577 within the kinase domain is required for maximal FAK catalytic activity, whereas the binding of FAK-family interacting protein of 200 kDa (FIP200) to the kinase region inhibits FAK catalytic activity. FAK phosphorylation at Tyr925 creates a binding site for GRB2.
3D structures of focal adhesion kinase
References
1.
2.
3.