User:Meng Han Liu/DNA termination of replication in E. coli & B. subtilis
From Proteopedia
Contents |
Introduction
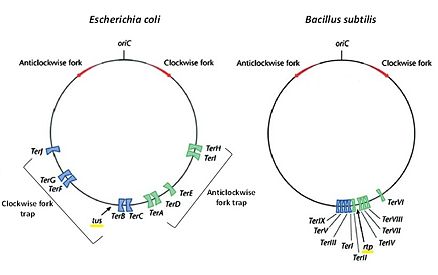
In prokaryotes such as E. coli and B. subtilis, chromosomal DNA exists in a circular fashion whereby DNA replication takes place at a common origin (oriC) [1]. Two replication forks move bidirectionally from oriC to replicate DNA until they eventually meet, and the forks fuse with one another to form two circular daughter chromosomes [2]. The region where the two replication forks meet is defined as the “terminus region”, located roughly opposite of oriC [3]. Bacteria use a “replication fork trap” system for successful termination of replication and fork fusion. This requires two factors:
- DNA terminator (Ter) sites
- A specific terminator protein that can bind Ter
DNA terminator (Ter) sites
Ter is a short consensus DNA sequence (around 20 base pairs long) that enables binding of its cognate terminator protein in order to arrest or halt replication fork progression in a polar manner i.e. it blocks replication fork coming in one direction (the non-permissive side) but allows passage when replication fork approaches from the other direction (the permissive side) [5]. In both E. coli and B. subtilis, multiple Ter sites are organized into two subgroups that flank the terminus region. Since replication fork arrest is unidirectional, Ter sites are distributed so that one subgroup only arrests the clockwise-moving fork while the other subgroup only arrests the anti-clockwise moving fork [4]. A suggestive reason for the presence of multiple Ter sites is to act as a safety measure to ensure termination of replication and fork fusion occur within the terminus region even if one of the replication forks managed to precede the innermost Ter sites.
DNA terminator proteins
DNA terminator proteins are proteins that can recognize and bind Ter DNA to form a complex in order to achieve polar trapping of replication forks [4]. In E. coli, this protein is called Tus (terminus utilization substance) whilst in B. sutilis, it is called RTP (replication termination protein). Based on experimental data using mutated Ter sites and DNA terminator mutants suggests that both protein-DNA (terminator-Ter) and protein-protein (terminator-replisome) interactions are important for successful polar fork arrest [6].
Biological roles of replication fork traps
As a matter of fact, it is not entirely essential to have replication fork traps in E. coli and B. subtilis to terminate DNA replication. This is because no deleterious effects were observed with respect to tus and rtp gene deletion experiments [4]. Therefore one possible reason of having replication fork traps is to reduce collisions of DNA replication and transcription apparatus as most genes are oriented in the origin to termination direction. Another role which replication fork traps may perform is to prevent over-replication of the chromosome by ensuring the two opposite replisomes dislodge within the termination complex.
In E. coli: Tus-Ter complex
Ter sites
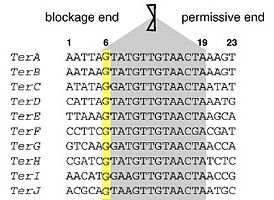
There are ten functional Ter sites (TerA-J) in E. coli, each 23 base pairs in length and are arranged in two opposite subgroups of five. No sequence symmetry or direct repeats occur across all Ter sites. For this reason, along with the fact that its cognate DNA terminator protein partner – Tus is asymmetric in nature, enables Tus to bind as a monomer. The core sequence of Ter is between base positions 6-19 and in particular, the G-C base pair at position 6 is strictly conserved [1][7]. These consensus sequences are important in Tus-Ter complex formation.
|
Crystal structure of Tus when bound to Ter
The crystal structure of the Tus-Ter complex was first unraveled by Kamada and co-workers [5]. Tus is a 36 kDa protein that binds Ter as a monomer. It consists of two asymmetrical domains: a larger and a smaller . Both domains are classified as α+β structures, made up of α-helices (αI-V) and β-sheets (βA-O), and are linked by 4 long loops (L1, L2, L3 and L4) that separate in between. Ter binding motif occurs in the interdomain of Tus which connects between the N- and C- terminal domains. It composes of two twisted antiparallel β strands (βF-βG and βH-βI) containing lots of basic residues, hence is positively charged. This large interdomain cleft is responsible for base specific recognition of Ter and intercalates tightly into the major groove of DNA. Furthermore, asymmetry in Tus binding gives rise to polar replication fork arrest. α-helices protruding at two sides are biased and mainly make contacts at the . This helps to protect the interdomain β cleft from direct contacts with replisomal proteins, which would be enough to displace Tus at the . Overall Tus embraces 13 base pairs of the DNA duplex through sugar-phosphate backbone contacts mediated by hydrogen bonds (H-bonds) or van der Waal interactions, as well as base contacts via H-bonds responsible for Ter recognition. Whilst the α-helical regions clamp the DNA at a girth-like manner, Tus is stabilized by high affinity binding of the interdomain.
Mechanism of polar fork arrest
Two models were proposed for halting replication fork movement at the non-permissive or blockage end [5]:
- The “interaction” model
- The “clamp” model
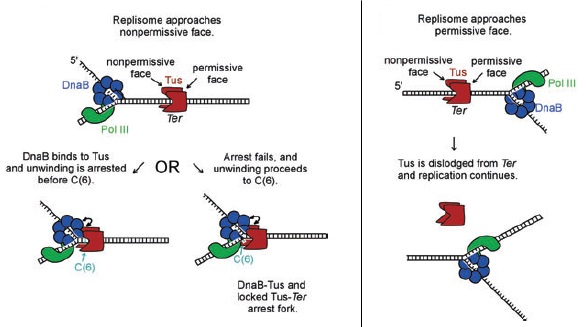
The "Interaction" model
This model encapsulates the idea that since helicase DnaB is the first replisomal protein to encounter the Tus-Ter complex as it unwinds DNA in the 5’-3’ direction on the lagging strand, protein-protein interactions between DnaB and Tus may be involved in replication fork arrest [7]. In fact, based on site-directed mutagenesis studies, this special contact occurs at the of Tus [8]. Besides, specificity of binding is elicited as some helicases in E. coli such as Rep is not blocked by Tus at the non-permissive end [6].
The "Clamp" model
The model as the name suggests, refers to phosphate backbone contacts of Tus via α-helical regions of N- and C-terminal domains that completely surround Ter at the non-permissive face,and this tight binding forms a physical barrier for stalling helicase DnaB thus fork progression [5]. Also, the idea was put forward that N-terminal α-helices can tangle the unwound 3’-5’ DNA which can strip off DnaB.
Furthermore based on base substitution experiments of Ter sites, it is demonstrated that exposure of the flipped Cytosine at position 6 of Ter - C(6) due to DnaB unwinding activity leads to binding within a hydrophobic pocket between Ile79 and Phe140 of Tus, forming a [7] which has a even higher binding affinity for Ter than normal double-stranded Ter binding. This conformation further enhances the stability of polar fork arrest and may act as a back-up system in case e.g. a DnaB-Tus-Ter arrest mechanism fails [6].
Although there is evidence to support either model, possibly a more feasible and logical mode of replication fork arrest in E. coli is to employ both models as a “fail-safe mechanism” [6]. As the replication machinery approaches the Tus-Ter complex on the non-permissive side, interactions between DnaB and Tus should be sufficient to halt replisome progression and this serves as the primary fork arrest mechanism. In case where this first blockage fails, further unwinding of DNA into C(6) by DnaB leads to lock complex formation, giving Tus a second attempt to stop the replication fork.
On the other hand, at the permissive or passage end of Tus, absence of a α-helical barrier [2] as well as strand separation by DnaB causes progressive loss of Tus-Ter contacts [7]. As a result Tus can easily dissociate and the replisome passes through the Ter site.
In B. subtilis: RTP-Ter complex
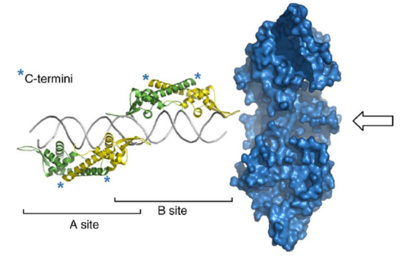
Ter sites
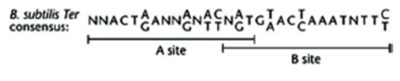
9 Ter sites (I-IX) are arranged into two groups: 4 to arrest the clockwise replication fork and 5 for the anti-clockwise fork [9].The Ter sites are 30 bp sequences comprising two imperfect inverted 16 bp repeats called the A and B sites [9]. The two sites overlap at a trinucleotide sequence that is highly conserved across all the Ter sites.
Two homodimers of RTP bind to A and B sites cooperatively but the binding to the two sites is not equivalent. B site has a higher binding affinity for the RTP dimer and has to be filled before A site [9]. The different hydrogen bonding sites and nonbonding contacts between the two sites and RTP were suggested to underlie the difference in binding affinity [10]. When both sites are filled, the affinity of RTP is higher due to cooperative binding interactions. A replication fork is only arrested when it is approaching from the non-permissive direction (proximal to B site).
|
Crystal structure of RTP
The first crystal structure presented by Bussiere et al suggested RTP binds to DNA as a symmetric homodimer of 29 kDa [11]. This was further confirmed by structural study using RTP.C110S mutants in which the lone Cysteine residue is replaced by Serine and the adapted symmetric TerI B site (sRB) DNA that differ to native B site (nRB) by three bases [10].However, a more recent study carried out by Vivian et al showed that crystal structure of the complex formed between RTP.C110S and the native nRB is asymmetric, consistent with an asymmetric binding mode in solution [9].
RTP belongs to the α+β protein folding class and each monomer contains four α-helices, three β-sheets and an unstructured N-terminal [11]. It has the classic winged-helix motif where “wings” project up or down from the loop between β2-β3 strands [9] and displays a unique mode of binding to DNA through interactions with both major and minor grooves [10].The N-terminal halves of of the dimer bind specifically to successive major grooves of DNA while the The β2-loop-β3 motif interacts with adjacent minor groove phosphate backbone [10]. The long C-terminal α helices of two monomers associate to form an antiparallel coiled-coil structure which defines the dimerisation domain [11].
Mechanism of polar fork arrest
RTP-DNA interaction
As RTP was thought to bind to Ter site in a symmetrical conformation, the polarity of replication fork arrest was proposed to arise from the differential binding affinity and cooperative binding to A and B sites (Differential affinity model)[9]. The binding of RTP to B site is strong enough to block replisome progression when A site is also filled whereas the weakly bound A site dimer will be displaced by advancing helicase [4]. This displacement disrupts the cooperative binding between the two sites and hence the weakened RTP-B site complex will allow passage of the replication fork. Another model suggests that the binding of RTP to Ter site will result in significant conformational change, rendering the RTP-Ter complex structure asymmetric (conformational change model)[9]. The two mechanisms are not mutually exclusive and may both account for the polarity of fork arrest. A recent crystal structure revealed that RTP is asymmetric in complex with DNA and the binding of RTP will result in distortion (bending) of upstream DNA [9]. This will facilitate further studies to provide insights into the basis of the polar action.
RTP-replisome interaction
In the past decade, a number of studies have demonstrated that the contacts between RTP and DNA is not sufficient to block replication fork progression, suggesting that asymmetric interactions between RTP and helicase is necessary for polar fork arrest [6].Gautam et al showed that a very instable RTP-Ter complex at check point Ter site was able to arrest replication fork in vivo [12]. Duggin et al demonstrated that the ability of fork arrest does not strictly correlate to RTP-DNA binding affinity, suggesting a strong DNA-protein interaction is not obligatory for fork arrest [13]. Another study revealed that fusion of peptides to the putative helicase interacting domain results in significantly reduced fork arresting efficiency without affecting DNA binding affinity [14]. It was also found that a mutant form of RTP with amino acid substitution at Tyr33 within the helix contact domain is not able to arrest replication fork, indicating the RTP-helicase interaction plays essential roles in mediating fork arrest [15].It is believed that interaction beween RTP and replisome is important for RTP function. This is achieved either the by highly specific surface residue interactions or secondary/tertiary structural complementarities [12].
Summary
In summary, the termination sequences and replication terminator protein structures are dissimilar in E. coli and B. subtilis, indicating independent origin of evolution. In spite of this, they both employ a replication fork trap mechanism in which high affinity binding of terminator protein to DNA through positively charged basic residues and terminator protein-replisome contact are required to achieve replication termination at defined terminus region in a polar manner.
| E. coli | B. subtilis | |
|---|---|---|
| Number of Ter sites | 10 | 9 |
| Size of each Ter site (bp) | 23 | 30 |
| Symmetry of Ter sites | asymmetric | peudo-symmetric
(A and B sites) |
| Name of the terminator protein | Termination utilisation substance (Tus) | Replication terminator protein (RTP) |
| Size of this protein (kDa) | 36 (monomer) | 29 (dimer) |
| Protein description | asymmetric helix clamp | winged-helix |
| Main secondary structures | α+β | α+β |
| Stoichiometry of protein-Ter binding | one monomer binds each Ter site | two dimers bind each Ter site |
| Key Ter binding motif | interdomain cleft | recognition helix (α3) |
| Groove of DNA insertion | major | major and minor |
| Basis of polarity in fork arrest |
|
|
| Mechanism of fork arrest |
| both RTP-Ter and RTP-replisome contacts involved |
References
- ↑ 1.0 1.1 1.2 Duggin, I.G. and S.D. Bell, Termination Structures in the Escherichia coli Chromosome Replication Fork Trap. Journal of Molecular Biology, 2009. 387(3): p. 532-539
- ↑ 2.0 2.1 Wake, R.G. and G.F. King, A tale of two terminators: crystal structures sharpen the debate on DNA replication fork arrest mechanisms. Structure, 1997. 5: p. 1-5
- ↑ Duggin, I.G., Wake, R. Gerry, Bell, Stephen D. Bell and Hill, Thomas M., The replication fork trap and termination of chromosome replication. Molecular Microbiology, 2008. 70(6): p. 1323-1333
- ↑ 4.0 4.1 4.2 4.3 4.4 Duggin, I.G. and J.A. Wilce, Termination of replication in bacteria. eLS2005: John Wiley & Sons, Ltd
- ↑ 5.0 5.1 5.2 5.3 Kamada, K., et al., Structure of a replication-terminator protein complexed with DNA. Nature (London), 1996. 383(6601): p. 598-603
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Kaplan, D.L.a.B., D., Mechanisms of polar arrest of a replication fork. Molecular Microbiology, 2009. 72(2): p. 279-285
- ↑ 7.0 7.1 7.2 7.3 Mulcair, M.D., et al., A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. Cell, 2006. 125(7): p. 1309-1319
- ↑ Mulugu, S., et al., Mechanism of termination of DNA replication of Escherichia coli involves helicase-contrahelicase interaction. Proceedings of the National Academy of Sciences of the United States of America, 2001. 98(17): p. 9569-9574
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 Vivian, J. P., Porter, C. J., Wilce, J. A. & Wilce, M. C. J. An Asymmetric Structure of the Bacillus subtilis Replication Terminator Protein in Complex with DNA. Journal of Molecular Biology 370, 481-491, doi:10.1016/j.jmb.2007.02.067 (2007)
- ↑ 10.0 10.1 10.2 10.3 10.4 Wilce, J. A. et al. Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. Nat Struct Mol Biol 8, 206-210 (2001)
- ↑ 11.0 11.1 11.2 Eli Bussiere, D., Bastia, D. & Stephen White, W. Crystal structure of the replication terminator protein from B. subtiiis at 2.6 Å. Cell 80, 651-660, doi:Doi: 10.1016/0092-8674(95)90519-7 (1995)
- ↑ 12.0 12.1 Gautam, A. & Bastia, D. A replication terminus located at or near a replication checkpoint of Bacillus subtilis functions independently of stringent control. Journal of Biological Chemistry 276, 8771-8777 (2001)
- ↑ Duggin, I. G., Matthews, J. M., Dixon, N. E., Wake, R. G. & Mackay, J. P. A Complex Mechanism Determines Polarity of DNA Replication Fork Arrest by the Replication Terminator Complex of Bacillus subtilis. J. Biol. Chem. 280, 13105-13113, doi:10.1074/jbc.M414187200 (2005)
- ↑ G. Duggin, I. DNA Replication Fork Arrest by the Bacillus subtilis RTP-DNA Complex Involves a Mechanism that Is Independent of the Affinity of RTP-DNA Binding. Journal of Molecular Biology 361, 1-6, doi:DOI: 10.1016/j.jmb.2006.06.013 (2006)
- ↑ Gautam, A., Mulugu, S., Alexander, K. & Bastia, D. A Single Domain of the Replication Termination Protein of Bacillus subtilis Is Involved in Arresting Both DnaB Helicase and RNA Polymerase. J. Biol. Chem. 276, 23471-23479, doi:10.1074/jbc.M009537200 (2001)
