Molecular Model:
We'll start with a simple ball-and-stick representation of the protein. This shows all of the atoms that make up the protein and the bonds between them.
BACKBONE:
The ball-and-stick view shows us all the atoms, but if we're mainly interested in the overall structure of the protein, this can be too much detail.
This next veiw takes us right down to a minimal representation that simply traces the of the protein. The backbone includes the peptide linkages between each amino acid, along with the alpha-carbon atoms to which the side chains are attached. Notice that helical regions can now easily be seen.
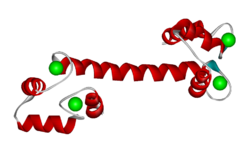
SECONDARY STRUCTURE: This is shown more clearly by a . The computer calculates where regions of secondary structure occur and draws them in cartoon-style 'ribbons'.
The α-helical region is now clearly defined, and there are also regions of β-structure.
Colour key:
Alpha Helices,
Beta Strands .
The short anti-parallel beta-sheet between the adjacent EF hand loops are observed in calmodulins from various species.
Calcium Binding
CALCIUM IONS:
In each EF hand loop, the Ca2+ ions are bound by amino acid residues in and near the loops.
The structure shown here has four bound. In this condition, the protein adopts the extended structure shown. The EF hand-forming helices are bent away from the long linking helix, revealing hydrophobic residues and exposing the linking chain.
CO-ORDINATING RESIDUES:
To illustrate how Ca2+ is bound, this display shows the one of the Ca2+ ions.
to see this more clearly.
CO-ORDINATING ATOMS:
To highlight the atoms that co-ordinate the Ca2+ ion, we can now enlarge those that are close (within 2.7 Å). This shows that atoms form the calcium co-ordination shell. Five are contributed by the side chain carboxyl groups of Asp and Glu and a sixth by the peptide carbonyl of Gln. The seventh oxygen is provided by an associated water molecule.
Binding to target proteins
ACTIVE & INACTIVE CALMODULIN:
At resting levels of cytosolic Ca2+ (~100 nM), calmodulin exists predominantly in the calcium-free form. This is called and its structure is more compact than the structure we saw earlier . Note the extended α-helix linking the two EF-hand-containing domains in the Ca-bound structure, which is interrupted in the . Here, the terminal helices are folded down concealing their hydrophobic surfaces and the central chain, which is not now α-helical along its whole length, is not exposed.
CALMODULIN INTERACTS WITH ITS TARGET:
The Ca2+-bound form of calmodulin with its exposed hydrophobic surfaces that you have already observed can . It does this by wrapping around a specific sequence on the target molecule, which is then forced into an α-helical structure.
The target molecule here (shown in blue) is the calmodulin-regulated enzyme, myosin light chain kinase. Only a short sequence from this protein, the calmodulin binding domain, is shown.
In this view, are coloured in order to highlight the hydrophobic interior of the molecule, which forms the binding site for the myosin light chain kinase calmodulin binding domain.
Hydrophobic, Polar