User:Mina Schneider/Sandbox
From Proteopedia
Trichosurin : a Possum Milk Whey Lipocalin Protein
|
The common brushtail possum (Trichosurus vulpecula) belongs to the metatherians, a mammalian clade, which diverged from placental mammals (like humains) approximately 130 million years ago. It includes all mammals with an abdominal pouche. Indeed, metatherians have invested in lactation (secretion of milk from the mammary glands), as opposed to placentation, as a way of rearing their young. Reproduction in Trichosurus vulpecula is typified by a short gestation (17 days) followed by a prolonged period of lactation (>200 days). Lactation is divided into at least three distinct phases between which milk composition changes dramatically [1], going along with a unique biology of the early development of young possum.
Trichosurin is one of the three predomiant lipocalins found in the milk of Trichosurus vulpecula. And its high level of conservation in species that diverged approximatively 80 million years ago, due to continental separation, suggests that the function of trichosurin is critical in metatherian lactation.
Lipocalins are a large family of extracellular proteins that diverse in sequence but are structurally homologous. They bind and transport small hydrophobic molecules in a central hydrophobic pocket. The lipocalin family is defined by a common fold: an eight-stranded anti-parallel β-barrel with variable loop regions flanking and enclosing the top and bottom of the barrel, to form a hydrophobic pocket.
Contents |
Structural highlights
Trichosurin consists of 165 amino acids.
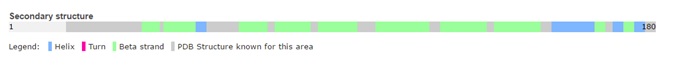
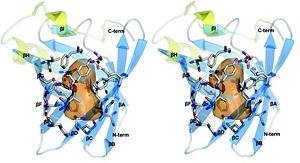
The two structures of trichosurin at both pH values 4.8 and 8.2 are very similar, with a root mean square difference in Cα atom positions of 0.54 Å over residues Leu24 to Cys166. The overall structural fold of trichosurin is an , anti-parallel β-barrel (in blue in Figure 2) with a near the C-terminus (in yellow in Figure 2) which is common to all lipocalins. An extended loop region between β-strands A and B forms a which, together with residues on the between β-strands E and F, close one end of the barrel. Residues which are N-terminal to β-strand A pack against residues in the bottom of the barrel, effectively sealing the other end of the barrel. A , the last major conserved feature of the lipocalin fold, is formed between Cys73 and Cys166 and ties the C-terminus to β-strand D.
Trichosurin forms an unusual dimer. Trichosurin, of molecular mass approximatively equal to 38 kDa, has 905 Ų of surface area per monomer buried by dimerization. This represents 11.8% of the monomer surface. The core of the dimerization interface is made of the ring to ring association of . The aromatic interaction is further complemented with the hydrophobic burial of the side-chains of . Two interactions between Arg150 and Asp98 also contribute to the stability of the dimer along with numerous inter-molecular hydrogen bonds. At least fifteen water molecules are present within the dimer interface in the structure at pH 4.6. Its dimerization mode is conserved between both structures at pH 4.6 and pH 8.2. The orientation of the Tyr94 side chain does however change position in the higher pH structure, leading to a tighter closing of the barrel in this area of the pH 8.2 structure when compared with the main barrel.
The internal topology of the hydrophobic pocket is a key factor in the type and selectivity of binding, as is access to the binding pocket which is usually controlled by the loops formed by the residues between beta-strands at one end of the lipocalin beta-barrel.
Internal topology: A ligand-binding cavity
The trichosurin-binding cavity is a hydrophobic pocket with approximate dimensions of 11× 6×6 Å (orange shape in Figure 2). The internal molecular surface is 425 Ų and encloses a total volume of ∼464 Å3. The cavity is constricted in the centre by the side chains of which protrude into the pocket.
Three asparagine residues, Asn49, Asn97 and Asn127 lie in the cavity and offer hydrogen-bonding sites for potential ligands.
A few water molecules bind inside the barrel, near the top of the hydrophobic pocket. They form hydrogen bonds with the carbonyl oxygen of the amino acids. Those water molecules found within the predominantly non-polar pocket bring along the formation of water mediated ligand-to-protein hydrogen bonds [2].
Thus, the central pocket is the major ligand-binding site of trichosurin.
The N-terminus
In lipocalins the N-terminus region of the amino acid chain helps to close the bottom end of the barrel, by conformational change linked to pH. In trichosurin, none of this is observed; the bottom of the barrel is effectively closed by . The structure at pH 8.2 further forms a classic lipocalin-like 310 helix with the amino acids Trp21, Glu22, Gln23 and Leu24. This protects the hydrophobic residues from the aqueous environment and plays a stabilizing role in this region of the structure. At this stage, the 20 first residues of the N-terminus chain couldn’t be determined, consequently it is not possible to assign them a structure or a role; however, they may perform a specific function such as a receptor-binding interaction.
Binding ligands
Two ligands have been found successfully binding into the pocket of trichosurin: 2-naphthol and 4-ethylphenol.
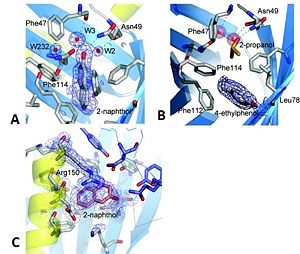
The 2-naphthol ligand binds to the top and centre of the pocket and makes a hydrogen bond to an internal water molecule, which in turn, is hydrogen-bonded by Phe47 O, Asn127 OD1 and Asn49 ND1 (see Figure 3A). Despite the presence of the ligand, the barrel remains completely closed. The binding of this ligand has resulted in a minor side chain rearrangement inside the binding pocket. The plane of the aromatic ring of Phe114 is rotated such that a small cavity is formed between the ring and the amide side chain of Asn127 and this is occupied by a water molecule. The water molecule accepts a hydrogen bond from the amide nitrogen but is also close enough to the aromatic ring to form an unconventional hydrogen bond by donation to the delocalized electron density of the aromatic ring plane.
4-ethylphenol binds in the lower of the two lobes of the binding pocket forming a hydrogen bond to Leu78 O. The axis of the ligand is oriented perpendicular to the barrel axis (see Figure 3B). With the ligand in this position the trichosurin-binding pocket is only partially filled and in this structure another molecule is able to occupy the upper lobe of the binding pocket. The trichosurin barrel is again completely closed. In addition to the interactions of a ligand with the inside of the trichosurin barrel, the 2-naphthol binds into the trough formed by the external barrel wall, the N-terminal helix and the dimer interface (see Figure 3C); this trough being enclosed on a fourth side by the side chain of Arg150. This second, externally binding site makes mainly hydrophobic interactions, so as a favourable aromatic interaction with the delocalized electrons of the guanidine head group of Arg150. Binding to this site implies that the ligand remains partially solvent exposed with 6–12 Ų accessible to the bulk solvent. The occupancy for this second binding site has been refined at 0.7 and is lower than the one for the main binding site of 0.9.
Trichosurin in the possum's organism
The binding of those two molecules within the trichosurin-binding pocket reveals the preferential binding of trichosurin with small phenolic compounds. As told before, trichosurin is a highly conserved protein found in the milk of metatherians. Its biological role is not fully understood yet. However, based on the analysis of its structure, it’s possible to make some hypothesis about its function. The ligand specificity of trichosurin for phenolic compounds, odours molecules, leads to the suggestion that the protein could deliver attractants. Those would guide the foetus from uterus to nipple in the early stages of metatherian development. Though, trichosurin is present in the possum’s milk throughout the whole lactation period [3], so this would only be part of its role. A second hypothesis relies on the fact that metatherians are generally herbivores and that phenolic compounds are significant constituents in plants. Some of them are toxic feeding deterrents. Thus trichosurin would enable the possum to metabolize these compounds [4]. Furthermore, female possums are able to concentrate phenolic compounds in their milk to levels appreciably higher than those found in the blood plasma [5]. This provides the young with a source of phenolics to enable priming of the neonate liver in the production of detoxifying enzymes. The specificity for small phenolics is consistent with the hypothesis that trichosurin may facilitate the process of carrying secondary plant phenolics through the milk and help in transfer across the neonate gut.
The crucial next step in resolving trichosurin’s function is to discover its natural ligands.
References
Main reference:
R.P. Watson, J. Demmer, E.N. Baker, V.L. Arcus "Three-dimensional structure and ligand binding properties of trichosurin, a metatherian lipocalin from the milk whey of the common brushtail possum Trichosurus vulpecula", Biochem. J. (2007) 408, 29–38
Further references:
- ↑ J Demmer, I.K. Ross, M.R. Ginger, C.K. Piotte and M.R. Grigor, "Differential expression of milk protein genes during lactation in the common brushtail possum (Trichosurus vulpecula)", Journal of Molecular Endocrinology (1998) 20, 37–44
- ↑ D.E. Timm, L.J. Baker, H. Mueller, L. Zidel, M.V. Novotny, "Structural basis of pheromone binding to mouse major urinary protein (MUP-I)", Protein Science (2001) 10, 997–1004
- ↑ J. Demmer, I.K. Ross, M.R. Ginger, C.K. Piotte and M.R. Grigor, "Differential expression of milk protein genes during lactation in the common brushtail possum (Trichosurus vulpecula)", Journal of Molecular Endocrinology (1998) 20, 37–44
- ↑ M.D. Dearing, S. Cork, "Role of Detoxification of Plant Secondary Compounds on Diet Breadth in a Mammalian Herbivore, Trichosurus vulpecula", Journal of Molecular Endocrinology (1999) 25, 1205-1219
- ↑ D.G. MacLennan, L.M. Blume, D.E. Johnson, "Induction of phenol detoxifying microsomal enzymes in juvenile brushtail possums Trichosurus vulpecula (marsupialia) by phenols transmitted in the maternal milk", Toxicon (1983) 21, 261–264