User:Stefanie Seuß/Sandbox 1494
From Proteopedia
|
Contents |
Serum amyloid A SAA 3
SAA3 participates in the inflammatory acute-phase response along with other specific acute-phase proteins (APP).
Organism: Mus musculus. Function: Major acute phase reactant. Apolipoprotein of the HDL complex. Induction: Translated by mice lung cells after secretion of APP.
As a general introduction to SAA, it should be highlighted that the term SAA itself refers to a whole family of apolipoproteins (that is, a type of proteins capable of binding to lipids) with four isomorphs in humans: SAA1, SAA2 and SAA4, being SAA3 encoded by the human pseudogene Saa3. The fact that this proteins are studied in mice is due to the high preservation of their role intra-species. During the APR in humans, Saa1 and Saa2 mRNA is thought to be transcribed in the liver, while Saa3 mRNA is thought to be transcribed extra-hepatically and close to the pulmonary area. In fact, SAA3 is indeed found to be synthesised by lung cells in mice.
The function and biochemical properties of SAA is highly preserved in several studied organisms such as vervet monkeys, mice and humans. This phylogenetic conservation allows us to extrapolate the studies carried out in mice to the effects it might have in humans, thus being model organisms for studies concerning SAA.
SAA3 can be, consequentially considered as one major SAA isomorph involved in the pathological condition linking inflammation processes on pulmonary tissue to cardiovascular diseases. Scarce literature is SAA3-specific but at the same time, it shares core feautures with the rest of the SAA family, which can be used to describe this molecule appealing to the aforementioned highly-preserved functional similarities found intraspecies.
In opposite to the abundant expression level of SAA1, SAA2 and SAA4, Human SAA3 (hSAA3) seemed to be a pseudogene and thought to be not expressed at all. But hSSA3 was indetified in the hSAA2-SAA3 fusion transcripts of several human cell lines.
Function
The SAA family as a whole plays an important role in the immune system, however, its specific role remains unknown. SAA is an apolipoprotein, thus able to bind lipoproteins such as HDL. In the reverse cholesterol transport (RCT), this protein can displace Apo-A1 as the major HDL-binding apolipoprotein. Apo-A1-HDL complex formation is necessary in order to remove cholesterol from peripheral macrophages and transport it to the liver for excretion. The specific binding by competition to HDL might be due to the Apo-A1 and SAA structural similarities and the fact that both are coded by two proximal genes found on chromosome 11 on humans and on chromosome 7 in mice.
Disease
It is suggested that the existence of a dose-dependent relationship between the inhalation of organic or inorganic particles suspended in the air leads to an immunological acute-phase response (APR) in the lungs which itself may induce the development of atherosclerosis.
Whenever an exposure to particles small enough to enter the respiratory track occurs, and these reach the lungs, a systematic immunological response as the APR is triggered. During this process, around fifty different types of acute phase proteins (APP) are released. These pro-inflammatory mediators play a major role for cell signalling during immunological responses. As a general feature, the response begins by the secretion of IL-1A (interleukin 1α) by macrophages, followed by other cytokines such as IL-1B (interleukin 1β), IL-6 (interleukin type 6) and TNF-α (Tumour Necrosis Factor 1α). The plasma concentration of many of these cytokines is consistently measured among scientific literature as indicators of inflammation in both studies on human and mice. Later during the long-term response serum amyloid A (SAA) secretion is induced.
However, continuous exposure as that related to certain occupational activities (mining, construction, metallurgical industry, etc.) may turn this process into a chronic condition. The long-time presence of SAA in the blood due to a chronic response is what has been associated with the formation of plaques in the walls of the aorta, which in turn may lead to atherosclerosis. The mechanism by which high-SAA levels in the blood triggers the progression of plaque formation is related to the reverse cholesterol transport pathway (see Function).
Relevance
The lack of structural information is an obstacle in understanding of SAA-mediated amyloidosis and the potential development of effective therapies.
Atherosclerosis is a major risk condition involved in cardiovascular diseases. Because atherosclerosis is catalogued as a chronic inflammatory disease, clinical markers for inflammation can be used as indicators to diagnose individuals having a high risk of CVD. Amongst these markers, serum amyloid A (SAA) serves as an excellent inflammation indicator because of peak secretion levels induced 24 hours after exposure to stressors.
When interpreting blood analysis data it should be consireded that SAA exists both in an acute-phase form (A-SAA) and a constitutive form (C-SAA). The A-SAA form has the two predominant isoforms SAA1 and SAA2, with primary structures that are 93% identical (98 of 104 amino acids). SAA1 is the most abundant isoform in plasma, nevertheless the relevance of the other SAA family members, including SAA3 has recently started to be more studied.
Structural highlights
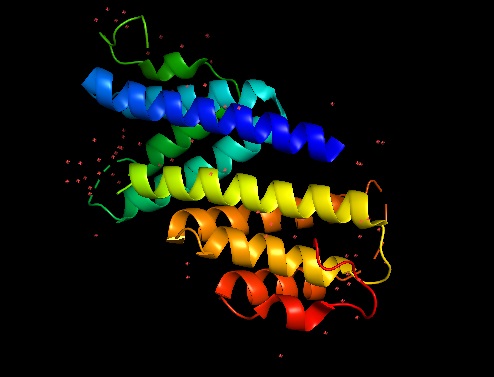
Apolipoproteic nature of the protein: abundant alpha helix domains and hydrophobic residues. Composed of 2 chains (2:A=B). 198 amino acid alpha carbons are present in the model. No attached nucleic acids are found. Lacking structural information: 10 Missing Residues including 2- charged amino acids. 1,688 atoms. 4 elements: C, N, O, Se. No hydrogen atoms depicted. Ligands+ and Non-Standard Residues: 6 MSE (Selenomethionine).
Polymer: 1
Length: 104 residues
Chain Type: polypeptide(L)
Reference: UniProtKB (P04918)
Identical Chains
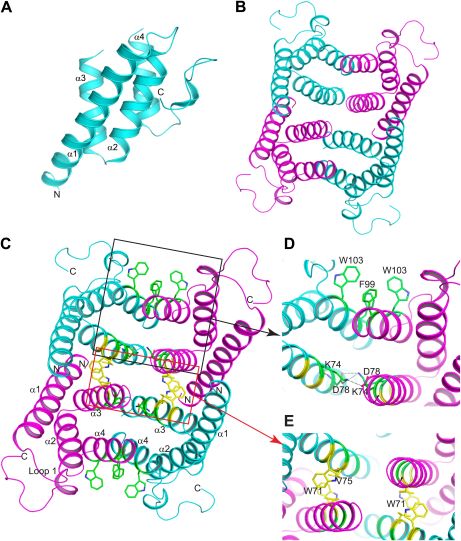
Mouse SAA3 forms a tetramer with a hydrophobic central channel. Crystallization of the protein lead to a P62 space group with two subunits in the asymmetric unit. Determination of the structure was performed using a 2Å resolution single-wavelength anomalous dispersion (SAD) phasing using a selenomethionyl-derivatived crystal. X-ray crystallography analysis reveals that mSAA3 is abundant in α-helical domains as it can expected from its primary sequence. Structural similarities can be observed between mSAA3 and human SAA1. Like the hSAA1, each of the two mSAA3 subunit consists of four α-helices, designated α1-4 when reading the chain from the N- to the C-termini, and form a cone-shaped four-helix with α1 being notably longer. The four helices form two sub-sets of antiparallel helices organised in pairs, α1-α2 and α3-α4, linked by short loop. The monomer is stabilized hydrogen bonding among neighbour residues and water molecules in the interior of the monomer. As for SAA1, the C-terminal tail wraps around the helix bundle making a numerous hydrogen bonds that add stability.
(A) Structure of the mSAA3 monomer (side view), with helices and termini labeled. (B) Top view of the tetrameric mSAA3 structure. Chains forming dimer pairs are colored cyan and magenta. In (C), helices α1-4 and the N- and C-termini of two monomers are labeled. Residues that make dimer contacts are shown as green sticks while residues involved in tetramer stabilization are shown as yellow sticks. (D and E) Magnified regions of a dimer interface (D) and tetramer interface (E) are shown.
References
- Bauman, R., et al. (2018). ‘’Systemic serum amyloid A as a biomarker for exposure to zinc and/or copper-containing metal fumes.’’ Journal of Exposure Science and Environmental Epidemiology, 28: 84–91.
- Coetzee, G.A., et al. (1986). ‘’Serum Amyloid A-containing Human High Density Lipoprotein 3.’’ The Journal of Biological Chemistry 261, 21: 9644-9651.
- Derebe, M.G., et al. (2014). Serum amyloid A is a retinol binding protein that transports retinol during bacterial infection. Elife, 3, e03206.
- Dong, Z., et al. (2011). ‘’Serum amyloid A directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice’’. Molecular medicine (Cambridge, Mass.), 17(11-12), 1357-64.
- Eklund, K.K., et al. (2012). ’’Immune functions of serum amyloid A.’’ Crit. Rev. Immunol 32(4):335-48.
- NCBI Genome Data Viewer. Mus musculus: GRCm38.p6. [Online] Retrieved from URL: https://www.ncbi.nlm.nih.gov/genome/gdv/browser/?context=genome&acc=GCF_000001635.26 [Last access: 13/11/2018].
- NCBI Genome Data Viewer. Homo sapiens: GRCh38.p12. [Online] Retrieved from URL: https://www.ncbi.nlm.nih.gov/genome/gdv/browser/?context=gene&acc=348 [Last access: 14/11/2018].
- Lu J., et al. (2014). Structure of native human serum amyloid A1. PDB 4IP9. [Online] Retrieved from URL: http://www.rcsb.org/structure/4IP9 [Last access: 12/12/2018].
- "Portions adapted from FirstGlance in Jmol": bioinformatics.org/firstglance