G15SecL05Tpc3
From Proteopedia
|
Contents |
Lyme Disease
Lyme disease is a bacterial infectious disease of the skin, joints, nervous system and heart dominantly caused by the spirochete Borrelia burgdorferi, transmitted to humans via the bite of the deer tick Ixodes scapularis (Becker et al, 2005). The importance of antibodies in controlling such spirochaetal infections was underscored by the discovery of two particular antibodies with distinctive bactericidal properties: the monoclonal antibodies CB2 and H6831 (Connoly et al, 2005). When directed against the C-terminus of the outer-surface protein Osp-B, these fragments are bactericidal even in the absence of complements or phagocytes (Sadziene et al, 1994).
Outer Surface Protein B (Osp-B) of the Borrelia burgdorferi Spirochete Bacterium Function
is a primary outer-surface lipoprotein molecule found in the Lyme disease spirochete Borrelia burgdorferi, a molecule essential for the survival of the bacterium. Since its primary function is to serve both as a site of antibody recognition and as the microvillar attachment to the Ixodes scapularis midgut, it is constitutively expressed (Becker, 2005). Osp-B is highly conserved among B. burgdorferi spirochetes, encoded on the linear plasmid “54” and is generally expressed by a common promoter with Osp-A (Neelakanta et al, 2007). Despite being documented about the structurally and functionally similar to Osp-A, the entirety of Osp-B’s functions and its independent roles in the life cycle of spirochetes ultimately remains unclear (Neelakanta et al, 2007). The formation of the OspB-CB2 and OspB-H6831 complexes were dependent upon a single lysine residue in Osp-B , for binding and thus leading to lysis of the outer membrane of the spirochete; the structural changes that result culminate into molecular instability and protease susceptibility that eventually lead to said lysis, though the exact physiological consequences of these changes are not yet fully sequenced nor understood (Connoly et al, 2005). These antibodies demonstrate enormous selective pressure; growth of Borrelia burgdorferi spirochetes in their presence generated escape mutants that lacked the critical Lys 253 amino acid on Osp-B for antibody binding, and were thus less infectious in experimental mouse models and in vitro experimental assays (Connoly et al, 2005). Variation in the synthesis of Osp-B and other outer surface proteins is the means by which B. burgdorferi evades the host immune system and adapts to various host micro-environments, such as those in the common tick vector. B. burgdorferi selectively expresses specific Osps in distinct phases of its life cycle and in specific tissue locations: expression of Osp-B is immediately turned on when the spirochetes enter and reside within the tick vector. However, during transmission from the tick vector to a vertebrate host, B. burgdorferi down-regulates OspB expression and up-regulates the expression of proteins such as OspC, DbpA, and BBK32. This selective gene expression of Osp-B (and additionally OspA) suggests that this protein may function during early spirochete colonization and persistence within the tick vector (Neelakanta et al, 2007).
Osp-B and H6831
Crystallization, binding and X-ray diffraction analysis of Osp-B along with a sampling of its complement-independent antibodies have shown that its structure is analogous to that of Osp-A, with a significant difference: within each borrelia species, Osp-A is largely invariant in amino acid sequence and antigenic reactivity, but Osp-B varies significantly (Becker et al, 2005). These variations inhibit the ability of scientists to develop Osp-B as a protective vaccine against Lyme borreliosis. Osp-B’s variations are primarily expressed as significant differences of reactivity with monoclonal antibodies and truncation of the C-terminus; this proves problematic for vaccine development since most protective antibodies targeted against Osp-A and Osp-B are generally directed toward this terminus region. Variations such as truncation in Osp-B can inhibit this process making the vaccine ineffective, though certain complement independent antibodies such as the samples examined in the crystallization process have been found to bind and lyse Osp-B in the absence of complement.
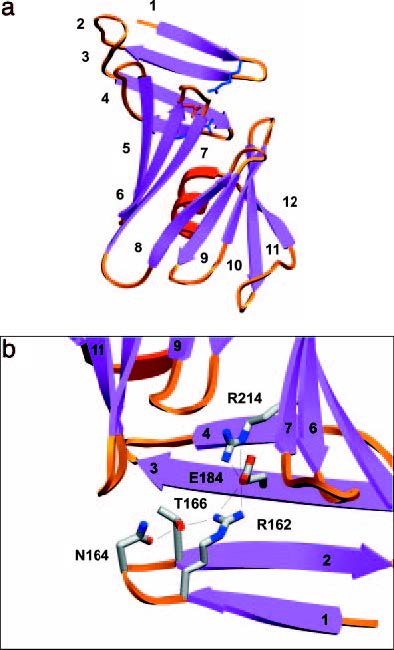
is a specific IgG class antigen binding fragment that has a series of significant residues that bind to the epitope on Osp-B and lyse it without the aid of a complement, resulting in a unique molecular interaction that causes a physical change in the shape of Osp-B upon binding. In order to better analyze the changes, crystallized structures of Osp-B and the Osp-B H6831 complex were taken and comparatively analyzed: X-ray diffraction showed that the structure of OspB-CT contains twelve anti parallel beta strands and a single alpha helix (Becker et al, 2005). Strands 1-4 form the Osp-B’s central sheet: this is a free standing sheet that is located at the N-terminus of the protein. The remaining strands 5-12 form two additional sheets that together with the alpha helix form a barrel-like domain. Analysis of the bound complex revealed that the central beta sheet is either destroyed or removed by proteolysis upon binding, as shown in the image above (Becker et al, 2005). Ultimately the structural changes to Osp-B appear to result only indirectly from the addition of Fab (Becker et al, 2005). Almost the entirety of this binding process is centered around distinct residues that were discovered due to comparative analysis of Osp-A and Osp-B’s crystal structures. A charged triad is formed in both structures whose united physical form presents itself as a favorable binding site. In Osp-B, this triad consists of amino-acid residues Arg-162, Glu-184, and (with additionally aiding the binding process though not part of the triad). These residues form a prominent cleft with density void space in between each other. This space is well suited for an all trans-structures to bind into, and such a structure was located on the crystallized Osp-B-H6831 bound complex. This complex is a combination of Osp-B-CT residues 202-296 and the Fabs’ heavy and light chains. A notable feature of the OspB-CT-H6831 interaction is the prevalence of aromatic residues (namely ) contributed by the Fab: these aromatic residues are the points at which Lys-253 on Osp-B guides itself to bind with . Specifically, Lys 253 wedges itself between residues Try-33 and of the H6831 heavy chain to be able reach the Glu-50 so the ion-pair binding between the two residues can occur (Becker et al, 2005). This interaction, along with an amine hydrogen bond and ion pair with a glutamate from the Fab heavy chain, a second hydrogen bond with a main chain carbonyl from loop "1" of Osp-B-CT, and a water mediated hydrogen bond to a histidine from the Fab heavy chain constitute the three main series of interactions in the OspB-H6831 bound complex. Lys-253 is the absolute key component of the formation of this bound complex; if Lys-253 were to be substituted with another amino acid or be missing entirely via external influential mutation, the binding could not occur (Connolly et al, 2005).
Multiple studies highlight selective pressures related to this residue: several mutated B. burgdorferi spirochetes have been shown to lack the Lys-253 residue entirely, though this form of the disease is significantly less pathogenic as a consequence. Further studies into this protein and complement-independent antibodies are important since as of now, vaccinations of Osp-B have proven unreliable due to its variable sequence and regional variations in its form making it an overall ineffective target for protective monoclonal vaccines, though direct bactericidal action of the signature H6831 and CB2 antibodies makes them as effective as some antibiotics (Becker et al, 2005). Creation of a proper vaccine has gradually grown in interest because the current antibodies created in the human body from Osp-A have been shown to have possible correlation with arthritis, an inflammatory joint disorder. In this regard, bactericidal antibodies to Osp-B may have in vivo relevance in late stage diseases and in vaccines for the elimination of spirochetes from ticks feeding on immune individuals (Escudero et al, 1997).
Osp-B/Antibody Bind Briefing (CB2)
Complement-independent antibodies have the ability to attack antigens directly in the absence of both complements or phagocytes. The exact mechanisms for these processes have not yet been clearly defined, though basic evidence shows that the attack of the complement-independent antibody creates a vesicle outgrowth that leads to an opening in the outer layer of the bacteria which leads to an osmotic lysis (LaRocca et al, 2009). The monoclonal CB2 antibody binds to the Osp-B protein of the spirochete bacteria: its high affinity toward the Borrelia's epitope binding site eliminates the bacteria accordingly. Selective pressure has led to multiple Borrelia bacterium escaping. This occurrence is due to possible point mutations at the C-terminus end of the Osp-B lipoprotein; namely, an absence of the Lys 253 amino acid which promotes the binding affinity. The antigen variations of borrelia inhibits the binding affinity of the antibody to the epitope located on the Osp-B (O'Hara et al, 2011).
Related Structures
- Crystal structure of the C-terminal fragment
- OspB-H6831 Complex
- CB2
- OspA complex with monoclonal antibody Fab
- OspC strain B31
Links
- Lyme Disease Center for Disease Control and Prevention, Lyme Disease Webpage
- Borrelia burgdorferi Borrelia burgdorferi spirochete
- Spirochetes
- Ixodida Ticks
- Lipoproteins
- Monoclonal Antibodies
- Arthritis Arthritis Foundation Webpage
Notes and Literature References
References:- 1. Becker M., Bunikis J., Lade B.D, Dunn J.J., Barbour A.G., Lawson C.L. “Structural Investigation of Borrelia burgdorferi OspB, a Bactericidal Fab Target” The Journal of Biological Chemistry; April 2005, pp. 17363- 17370
- 2. Connolly S.E., Benach J.L. “The Versatile Roles of Antibodies in Borrelia Infections” Nature Reviews: Microbiology, May 2005, pp. 411-420
- 3. LaRocca T.J., Benach J.L. 2008 “The Important and Diverse Roles of Antibodies in the Host Response to Borrelia Infections” Specialization and Complementation of Humoral Immune Responses to in Infection. Current Topics in Microbiology and Immunology: pp. 319
- 4. Escudero R, Halluska ML, Backenson PB, Coleman JL, Benach JL. "Characterization of the Physiological Requirements for the Bactericidal Effects of a Monoclonal Antibody to OspB of Borrelia burgdorferi by Confocal Microscopy". Infect Immun. 1997, pp. 1908-15
- 5. Sadziene A, Jonsson M, Bergström S, Bright RK, Kennedy RC, Barbour AG. "A Bactericidal Antibody to Borrelia burgdorferi is Directed Against a Variable Region of the OspB Protein". Infect Immun. 1994; 62(5): pp. 2037-45.
- 6. Neelakanta G, Li X, Pal U, et al. "Outer Surface Protein B is Critical for Borrelia burgdorferi Adherence and Survival Within Ixodes Ticks". PLoS Pathog. 2007, pp. 33
- 7. O'Hara N, LaRocca T, Spikes D, Miyazaki J, O'Neal M."Fundamentals of Scientific Inquiry in the Biological Sciences". Hayden-McNeil Publishing. Stony Brook University. 2011, pp. 97-117