Sandbox 156
From Proteopedia
Contents |
Chloramphenicol Acetyltransferase Type III
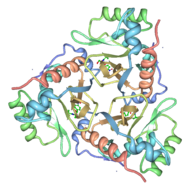
Chloramphenicol acetyltransferase type III (CAT III) is an enzyme which catalyzes the transfer of the acetyl group from Acetyl-CoA to hydroxyl groups of chloramphenicol. CAT III is a trimeric protein with a Mr of 25 000-kDa and a member of the actetyltransferase family of proteins.
Introduction
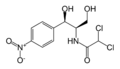
Found in bacteria, the CAT III enzyme is responsible for conferring resistance of the antibiotic chloramphenicol to the cell. Chloramphenicol arrests protein synthesis by binding to the bacterial ribosomes and causing the inhibition of peptidyl transferase activity[1]. However, when CAT III catalyzes the acetylation of chloramphenicol, the antibiotic can no longer bind to the ribosomes and is rendered inactive. The genes for the enzyme are commonly found on the plasmid of the bacteria and have been found in a number of bacterial species[2].
The multifunctional enzyme consists of three identical subunits with three active sites at the subunit interfaces[1]. A deep, hydrophobic pocket is formed at the interfaces, allowing for binding of the chloramphenicol substrate[3]. Binding of the second substrate, Acetyl-CoA, is accomplished by passing the molecule through a tunnel in the protein to the active site[3]. The active site of CAT III performs acetylations of chloramphenicol via a ternary complex mechanism and can accommodate the presence of the first intermediates quite well[4].
Reaction of CAT III
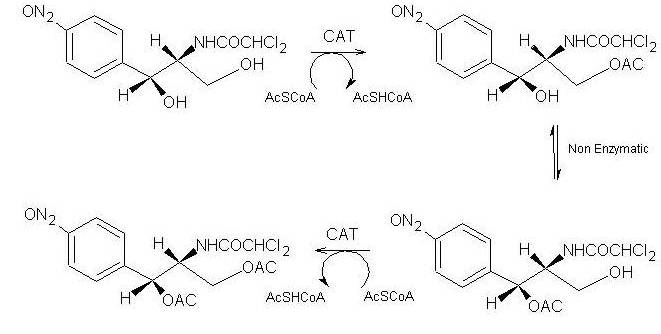
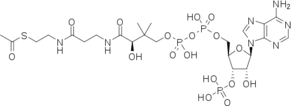
Both reactions take place in the active site of CAT III, where Acetyl-CoA is tunneled through from the opposing side of the trimer[3].
In the first step of the reaction, Histidine-195 abstracts a proton from the 3-hydroxyl of chloramphenicol, promoting a nucleophilic attack from the resulting oxyanion to the thioester bond of the Acetyl-CoA[4]. The intermediate produced, 3-acetylchloramphenicol, then rearranges non-enzymatically to 1-acetylchloramphenicol[4]. Regeneration of the 3-hydroxyl allows another round of CAT III catalyzed acetylation and a 1,3-diacetylchloramphenicol product is formed[4].
Structure
|
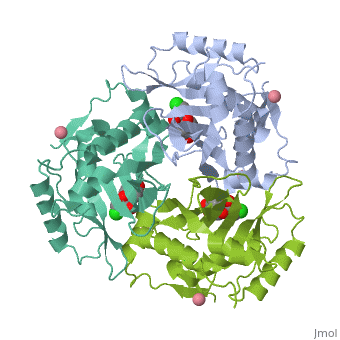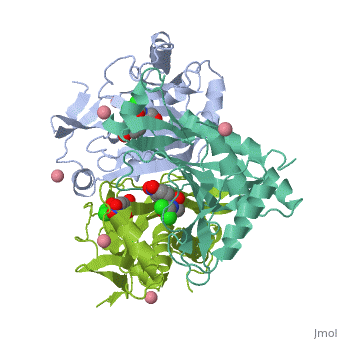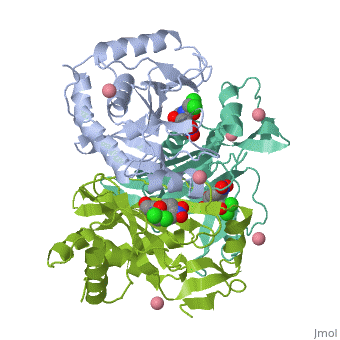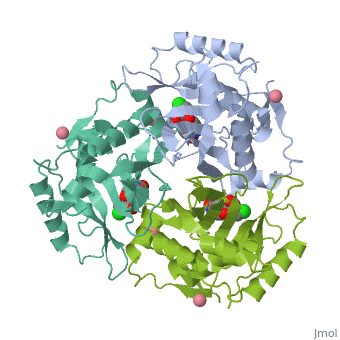
The general structure of CAT III is dominated by a six stranded antiparallel and 5 , stacked against the ends and face of the protein, forming a structure known as an "open-faced sandwhich"[3]. An extended β-strand forms an extension of the six stranded sheet to a seven stranded sheet that spans the interface of the subunit[3]. Three identical monomers associate to form the trimeric protein with two ions acting as cofactors[5]. The side chains of one subunit allow van der Waals interactions and two hydrogen bonds with chloramphenicol, causing binding of the substrate. The opposing subunit provides a histidine (His-195) residue essential for catalysis[6].
The Chloramphenicol Binding Site
The association of the monomeric subunits produces a well-defined pocket at the subunit interface, accommodating the chloramphenicol substrate and ordered water molecules [7]. The of CAT III is lined with hydrophobic residues, allowing van der Waals interactions and 2 hydrogen bonds to form betwen the eznyme and the substrate[3]. A third hydrogen bond is mediated through a and allows interaction between the the 1-hydroxyl of chloramphenicol and the hydroxyl of Tyr-174[4].
The Acetyl-CoA Binding Site
The binding of chloramphenicol blocks Acetyl-CoA from entering the active site from the , as illustrated[3]. Instead, the second substrate tunnels to the active site histidine through the from the opposite side of the trimer[1]. The amino acids lining the tunnel are also primarily hydrophobic producing close contact between the substrate and aromatic residues[8]. This tunneling allows the formation of van der Waals interactions between the adenine ring of acetyl-CoA with dimethyl groups of the pantetheine arm and hydrogen bonding of the ring with Tyr-56,Phe-96 and Phe-103, binding the substrate[3].
The Active Site
The active site catalyzes sequential acetylations of the chloramphenicol molecule. This is initiated by the deprotonation of the 3-hydroxyl of chloramphenicol by , which acts as a general base[4]. Deprotonation is due to the orientation of His-195, which brings the imidazole ring of histidine into contact with the chloramphenicol[1]. Numerous hydrogen bonds are present and, importantly, van der Waals interactions between the benzene ring of the residue and the imidazole ring[1]. This van der Waals contact may help stabilize side-chain orientations, promoting specificity in the reaction[1]. The acetyl group is already properly positioned after tunneling, and reacts readily without any major structural changes[3].
Structure used in the above scenes: 3cla
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Day PJ, Shaw WV. Acetyl coenzyme A binding by chloramphenicol acetyltransferase. Hydrophobic determinants of recognition and catalysis. J Biol Chem. 1992 Mar 15;267(8):5122-7. PMID:1544895
- ↑ Lewendon A, Shaw WV. Elimination of a reactive thiol group from the active site of chloramphenicol acetyltransferase. Biochem J. 1990 Dec 1;272(2):499-504. PMID:2268277
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Leslie AG, Moody PC, Shaw WV. Structure of chloramphenicol acetyltransferase at 1.75-A resolution. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4133-7. PMID:3288984
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Murray IA, Lewendon A, Williams JA, Cullis PM, Shaw WV, Leslie AG. Alternative binding modes for chloramphenicol and 1-substituted chloramphenicol analogues revealed by site-directed mutagenesis and X-ray crystallography of chloramphenicol acetyltransferase. Biochemistry. 1991 Apr 16;30(15):3763-70. PMID:2015231
- ↑ Gibbs MR, Moody PC, Leslie AG. Crystal structure of the aspartic acid-199----asparagine mutant of chloramphenicol acetyltransferase to 2.35-A resolution: structural consequences of disruption of a buried salt bridge. Biochemistry. 1990 Dec 25;29(51):11261-5. PMID:2271709
- ↑ Lewendon A, Shaw WV. Transition state stabilization by chloramphenicol acetyltransferase. Role of a water molecule bound to threonine 174. J Biol Chem. 1993 Oct 5;268(28):20997-1001. PMID:8407936
- ↑ Lewendon A, Murray IA, Shaw WV, Gibbs MR, Leslie AG. Evidence for transition-state stabilization by serine-148 in the catalytic mechanism of chloramphenicol acetyltransferase. Biochemistry. 1990 Feb 27;29(8):2075-80. PMID:2109633
- ↑ Lewendon A, Murray IA, Shaw WV, Gibbs MR, Leslie AG. Replacement of catalytic histidine-195 of chloramphenicol acetyltransferase: evidence for a general base role for glutamate. Biochemistry. 1994 Feb 22;33(7):1944-50. PMID:7906544
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |