To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
For more help, look at this link:
http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Cholix Toxin from Vibrio cholerae
Introduction
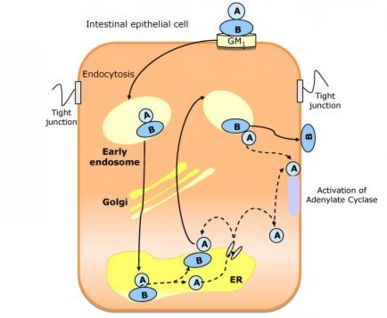
Cholix toxin (CT) is a class of protein exotoxin originating from the bacteria Vibrio cholerae. It is the third member of eEF2 specific ADP-ribosyltransferase toxins.[1] The toxin uses ADP-riboyltransferase to inactivate eukaryotic elongation factor 2 by transferring ADP-ribose from NAD+ which inhibits protein synthesis and causes cell death. This protein toxin has been known to cause disease in both plants and animals. Cholix is the causative agent of the disease Cholera. It enters eukaryotic cells through endocytosis. Once inside, the toxin transfers an ADP-ribose group to an Arg residue of the GTP binding protein G. This activates adenylate cyclase which leads to an increase amount of cAMP, causing a secretion of Cl-,HCO3-, and water from epithelial cells from the site of colonization [2]. The result is dehydration and loss of electrolytes in infected hosts. "Structurally, it is a three-domain A/B toxin. It consists of a receptor binding domain for receptor mediated endocytosis, a membrane translocation domain for the translocation to the host cytoplasm, and a catalytic domain" [3]
Structure
X-Ray Crystallography
"To observe the structure, the Cholix Toxin was crystallized by vapor diffusion against reservoirs containing 23% polyethlene gylcol-10,000, 7.5% ethylene glycol, and 0.1 m HERPES." [4] In addition, the reservoirs were kept at a of pH 7.5 and at a temperature of 19°C. About 40 µL of reservior solution containing 1.25 mM NAD+ solution was added to a 2 µL crystal containing group. The NAD+ was allowed to soak into the crystals for approximately 2-3 minutes. The crystals were then transferred to paratone-N for cryoprotection. Then to visualize it, a diffractometer was used.[5]
Protein Structure
The sequence of CT is 666 amino acids in length. It contains a total of four disulfide bonds which are located between Cys-43:Cys-47, Cys-240:Cys: 257,Cys-310:Cys-332, and Cys-426:Cys-433.[6]. The secondary structure of the Cholix Toxin consists of 11 and 4 The Cholix Toxin contains three different domains. Though it is not shown, the active site for this toxin is located at Glu-613. interactions with NAD+ include V360,T469,I470,V477,L492, and V500. There is also interactions between the stacking of the nicotinamide ring and Y493 and Y504.[7]
Molecule
"CT is a bipartite molecule belonging to the AB5 family, which also includes Shiga and pertussis toxins. CT combines one A active subunit and five identical peptides that is assembled into a highly stable pentameric ring named B subunit. The A subunit presents two domains:A1 and A2."[8] The B subunit serves to bind to GM1 ganglioside on the surfaces of intestinal epithelial cells.[9] Both of these subunits are highly essential for the functionality of this toxin.
Mechanism of Action
Basic Mechanism
The mechanism for the cholix toxin occurs as follows:
- The Cholix Toxin binds to the GM1 gangliosides of host cell membrane.
- Toxin gains access inside the cell through endocytosis.
- CT-GM1 complex forms.
- CT-GM1 complex travels through the Golgi cisternae and to the Endoplasmic Reticulum (ER).[10]
- A1 peptide of the toxin will unfold and dissociated from B pentamer.
- The unfolded A1 is translocated to the cystol and will eventually leave the membrane after translocation.
- A1 will move by diffusion to attack the adenylate cyclase complex.
- The B subunit does not move across the membrane, rather it remains bound to the GM1 receptors.
- The active subunit A1 uses its enzymatic ability to transfer ADP-ribose group to an Arg residue of the α subunit of the GTP binding protein Gs.[11]
- The Gs-α subunit becomes activated and breaks away from the Gs membrane-bound subunit and goes to attach itself to the catalytic site of the adenylate cyclase, activating this enzyme.
- The activation of adenylate cyclase causes increase in cAMP levels which inhibits Na+ and Cl- absorption and increases secretion of Cl- and HCO3- which leads to dehydration and loss of electrolytes in the infected host. The activation of adenylate cyclase also causes pore formation within the intestinal epithelial cell membrane which leads to cell death.[12]
ADP-ribosylation
Not only does Cholix use ADP-ribosylation to activate the Gs-α subunit of G-Proteins, but it also covalently transfers the ADP-ribose from NAD+ to diphthamide on eEF2 (Eukaryotic Elongation Factor 2) through nucleophilic substitution. This causes for the inhibition of protein synthesis and cell death.[13]
Medical Implications and Other Applications
- The Cholix toxin is the main virulence factor associated with V.cholerae pathogenesis. Cholera is a disease which can cause a heavy amount of diarrhea which leads to dehydration.Today, Cholera continues to be the leading cause of death in many third world countries since they are present in poorly treated water supplies."Patients can lose as much as 20 liters of fluid in 24 hours and more than 50% of them die if not treated"[14]
- Cholera toxin treatment activates the adenylate cyclase in intact HeLa cells. However, pretreatment of the cells with chemicals known to inhibit receptor internalization and lysosomal processing blocks the toxin activation. The agents found to inhibit the effect of cholera toxin include methylamine, ammonium chloride, chloroquine and dansylcadaverine. These chemicals act on the processing of the toxin subsequent to its binding and that internalization and lysosomal processing mediate the release of the active fragment from cholera toxin, which activates the adenylate cyclase system.
References
- ↑ Jorgensen, R., Purdy, A., Fieldhouse, R., Kimber, M., Bartlett, D., & Merrill, A. (2009). Cholix toxin, a novel adp-ribosylating factor from vibrio cholerae. Journal of Biological Chemistry, 283(16), 10671-10678.
- ↑ Jorgensen, R., Purdy, A., Fieldhouse, R., Kimber, M., Bartlett, D., & Merrill, A. (2009). Cholix toxin, a novel adp-ribosylating factor from vibrio cholerae. Journal of Biological Chemistry, 283(16), 10671-10678.
- ↑ Fieldhouse, R., Jorgensen, R., Lugo, M., & Merrill, A. (2012). The 1.8A cholix toxin crystal structure in complex with NAD+ and evidence for a new kinetic model. 1-14.
- ↑ Jorgensen, R. (Artist). (2008). PDB File Cholix toxin, a novel adp-ribosylating factor from vibrio cholerae.. [Web Graphic]. Retrieved from http://www.rcsb.org/pdb/explore/explore.do?structureId=2q5t
- ↑ Jorgensen, R., Purdy, A., Fieldhouse, R., Kimber, M., Bartlett, D., & Merrill, A. (2009). Cholix toxin, a novel adp-ribosylating factor from vibrio cholerae. Journal of Biological Chemistry, 283(16), 10671-10678.
- ↑ UniPro (2012, May 2). Cholix Toxin Precursor. Retrieved May 2, 20012, from http://www.uniprot.org/uniprot/Q5EK40.
- ↑ Fieldhouse, R., Jorgensen, R., Lugo, M., & Merrill, A. (2012). The 1.8A cholix toxin crystal structure in complex with NAD+ and evidence for a new kinetic model. 1-14.
- ↑ Valerio, E., Chaves, S., & Tenreiro, R. (2010). Diversity and impact of prokaryotic toxins on aquatic environments:a review. (2), 2359-2410.
- ↑ Valerio, E., Chaves, S., & Tenreiro, R. (2010). Diversity and impact of prokaryotic toxins on aquatic environments:a review. (2), 2359-2410.
- ↑ Valerio, E., Chaves, S., & Tenreiro, R. (2010). Diversity and impact of prokaryotic toxins on aquatic environments:a review. (2), 2359-2410.
- ↑ Valerio, E., Chaves, S., & Tenreiro, R. (2010). Diversity and impact of prokaryotic toxins on aquatic environments:a review. (2), 2359-2410.
- ↑ Valerio, E., Chaves, S., & Tenreiro, R. (2010). Diversity and impact of prokaryotic toxins on aquatic environments:a review. (2), 2359-2410.
- ↑ Fieldhouse, R., Jorgensen, R., Lugo, M., & Merrill, A. (2012). The 1.8A cholix toxin crystal structure in complex with NAD+ and evidence for a new kinetic model. 1-14.
- ↑ Valerio, E., Chaves, S., & Tenreiro, R. (2010). Diversity and impact of prokaryotic toxins on aquatic environments:a review. (2), 2359-2410.
|