Sandbox Reserved 489
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia Renin Renin, also known as angiotensinogenase, is an aspartyl protease and belongs to the protein family peptidase A1. Aspartyl proteases are endopeptidases that typically use two aspartate residues in the active site to specifically cleave peptide substrates using an acid-base hydrolysis mechanism. Mature renin circulates in the blood stream and contains 340 amino acid residues and has a mass of approximately 37 kDa. The function of renin is to cleave angiotensinogen to produce angiotensin I. Renin is secreted by the kidneys. The kidneys act both directly and indirectly to regulate arterial blood pressure and provide the major long term mechanism of blood pressure and control. The direct mechanism changes blood volume independently of hormones. When blood pressure and blood volume increase the kidneys can not filter all of the liquids and thus liquids are lost in the urine to decrease blood pressure and blood volume. The indirect mechanism, or the renin-angiotensin system (RAS), controls blood volume and blood pressure through renin and two forms of angiotensin. Renin is involved in the rate limiting first step of a cascade that eventually produces angiotensin II. The specialized granular cells of the juxtaglomerular apparatus secrete renin when stimulated by the macula densa when blood pressure or blood volume decreases. Renin circulating in the blood stream cleaves a small 10 residue portion of plasma protein angiotensinogen that is secreted by the liver. Cleavage of angiotensinogen produces the inactive precursor angiotensin I that is converted to angiotensin II by angiotensin-converting enzyme primarily in the lungs. Angiotensin II increases blood pressure in three ways.
Additionally, angiotensin II triggers the sensation of thirst. The release of renin into the blood stream ultimately raises blood pressure. The kidneys restore and maintain blood pressure homeostasis by regulating blood volume through the action of renin. Although blood volume varies with age, body size, and sex, renal mechanisms usually maintain it to 5 liters. Renin has been identified in many eukaryotic organisms including; humans, mice, marmosets, monkeys, chimpanzees, macaques, dogs, rats, frogs, and zebrafish. The function of renin in all organisms is similar, but the sequence and peptide length vary slightly. Renin is important clinically because renin inhibitors, such as aliskiren, can be used to treat hypertension.
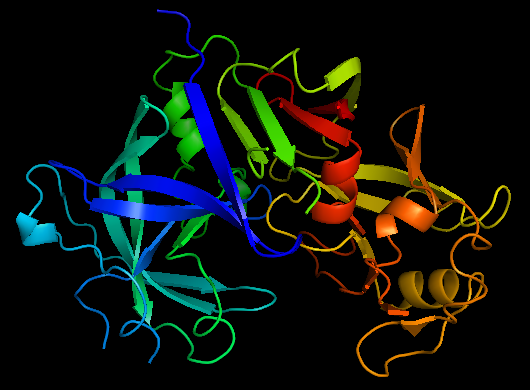
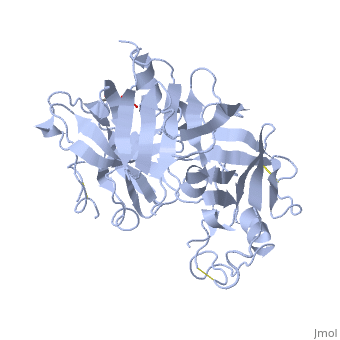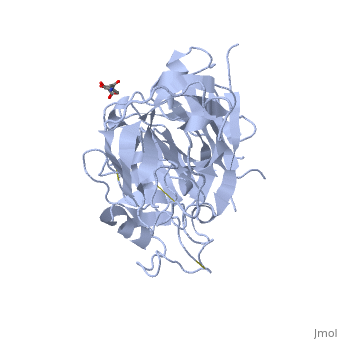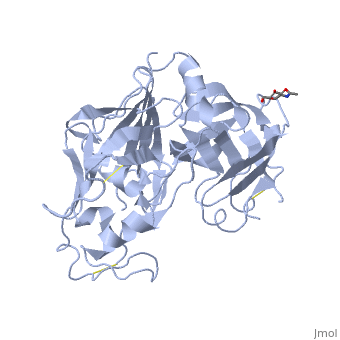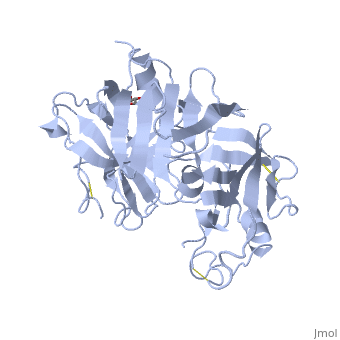
Structure
The precursor of renin is a 406 amino acid residue protein. are a signal peptide sequence and residues 24-66 are cleaved to produce the mature 340 amino acid residue . The secondary structural elements of renin include 29 , 3 , 4 , 310, and . The most impressive structural feature of renin is the antiparallel that forms the two similar lobes of renin. are located primarily on the outside and inside portions of renin respectively. The most important structure is the located in the active site that allows substrate binding. The active site of renin contains two essential . Renin has after each of the two aspartate residues. Renin also uses a , a β hairpin structure, that open and closes to uncover or cover the active site. Post translational modifications of renin include; precursor cleavage of propetide to produce active mature renin, disulfide bond formation, and glycosylation of certain residues. Disulfide bonds are formed to connect serine residues , , and . at positions 14 and 75 can be glycosylated. The asparagine residue at postion 75 is glycosylated (2-(acetylamino)-2-deoxy-A-D-glucopyranose) in mature renin whereas the residue at postion 14 is not glycosylated.[1] The structure of recombinant human renin has been solved using X-ray diffraction at 2.5 Å resolution.[2] Function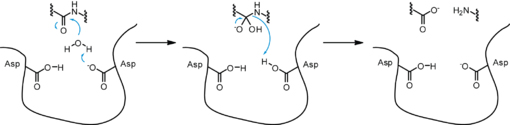 Aspartyl Protease Mechanism[3] Renin secretion is stimulated by a decrease in arterial blood pressure, a decrease in sodium chloride levels in kidney nephrons, or sympathetic nervous system activity. The substrate of renin, angiotensinogen, is a 452 amino acid residue in humans. Renin utilizes two aspartate residues in the to cleave the peptide bond between leucine and valine residues on angiotensinogen. Angiotensin I is an inactive short peptide of 10 amino acids that is produced by the renin cleavage reaction. The close proximity of the two aspartate allows the acid-base hydrolysis mechanism to cleave the peptide bond. Because the aspartate residues are close together one residue has a higher pKa and the other residue has a lower pKa. The mechanism of the catalysis is an acid-base transfer of water between the two aspartate residues. There is a water molecule associated with the two aspartate residues in the active site and also a water associated with the peptide bond that is cleaved. Initially one aspartate residue carbonyl is deprotonated and the other is protonated. The deprotonated aspartate removes a proton from water allowing the water to attack the carbonyl of the peptide bond in the substrate forming a tetrahedral oxyanion intermediate on the substrate. Rearrangement of the intermediate causes protonation of the amide on the substrate completing the cleavage reaction. A concerted mechanism of action has also been proposed that states the active site aspartate and water attack the scissle peptide bond in a single step with no covalent tetrahedral intermediate, while still forming the tetrahedral transition state. Renin can also bind the renin receptor ATPase H(+)-transporting lysosomal accessory protein 2 (ATP6AP2) to convert angiotensinogen to angiotensin I at a much faster rate.[4] Renin Inhibitors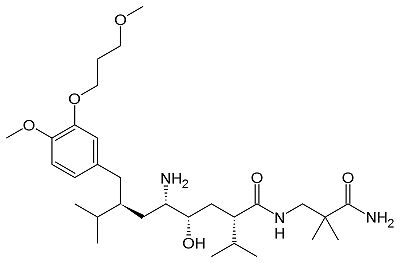 Aliskiren[5]
There are three generations of renin inhibitors. The first two generation molecules were peptide molecules. These peptide molecules were not specific or effective as renin inhibitors. Aliskiren, part of the 3rd generation, is a nonpeptide renin inhibitor. Small molecule nonpeptide inhibitors such as aliskiren have good pharmokenetics and are very specific for renin and not other protein peptidases. Advancements in crystallography and molecular modeling allowed the discovery of aliskiren. Aliskiren inhibits renin activity. Since renin is the rate limiting step of the RAS renin inhibition is a successful method to lower blood pressure. Aliskiren is a hydrophilic molecule. When bound to renin, aliskiren occupies the S1, S1', S2', and S3 hydrophobic regions of renin. Most importantly aliskiren occupies the S3SP region that is equally hydrophobic and hydrophilic and greatly increases binding affinity.[6] Aliskiren interacts with multiple residues in renin. The hydroxyl group hydrogen bonds both oxygens. The amine group hydrogen bonds carboxylic acid group of . The methoxy group in the S3 hydrophobic region hydrogen bonds to secondary amine group of . The amide group hydrogen bonds with the secondary amine of .[7] And the terminal amide hydrogen bonds with in the S2' hydrophobic pocket.[8] Aliskiren is approved by the Federal Drug Administration (FDA) to treat hypertension. However, there are certain drug combinations that can be dangerous in combination with aliskiren. The FDA announced on April 20, 2012 that patients with diabetes should not be prescribed drugs containing aliskiren in combination with angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Examples of drugs that combine aliskiren with other drugs include valturna, tekturna HCT, tekamlo, and amturnide. The drug combinations increase the risk of renal impairment, hypotension, hyperkalemia, and lack of efficacy in diabetes patients. The drug combinations should also be avoided in patients with renal impairments. The announcement was based on a Novartis-sponsored clinical trial called Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE). The FDA also stated that preliminary ALTITUDE data indicated an increase in cardiovascular risks such as stroke and death but further trial results are necessary to confirm these risks.[9] The structure of renin bound with the inhibitor aliskiren has been solved using X-ray diffraction at 3.0 Å resolution.[10] DiseasesRenal tubular dysgenisis is caused by defects in the renin gene. Renal tubular dysgenisis is an autosomal recessive disorder of renal tubular developement and is characterized by persistant fetal anuria and perinatal death. The RAS plays a crucial role in the developement of the kidneys during early fetal life. [11] Familial juvenile hyperuricemic nephropathy type 2 is also caused by defects in the renin gene. Familial juvenile hyperuricemic nephropathy type 2 is characterized by slowly progressive renal failure and anemia. The autosomal dominant disorder is caused by a deletion of leucine 16 or a mutation of leucine 16 to arginine. The mutations effect the hydrophobicity of the signal sequence and disrupt the proper transport of preprorenin into the endoplasmic reticulum and thus effecting prerenin processing. The mutatnt proteins are toxic and reduce the viability of renin expressing cells, eventually causing renal failure.[12] References
|