To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
For more help, look at this link:
http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase (CODH/ACS)
Introduction
Carbon monoxide dehydrogenase/acetyl-CoA synthase (CODH/ACS) is a bifunctional protein which acts as both an oxidoreductase and a transferase by reducing carbon dioxide to carbon monoxide (or the reverse oxidation of carbon monoxide to carbon dioxide) and then catalyzing the synthesis of acetyl-CoA from carbon monoxide, coenzyme A, and the methyl group of an corrinoid iron-sulfur protein. This enzyme plays a key role in the Wood-Ljungdahl pathway which is used by anaerobic, autotrophic bacteria such as Moorella thermoacetica (f. Clostridium thermoaceticum) and Clostridium ljungdahlii for gaseous carbon fixation. End products of the Wood-Ljungdahl pathway include cell biomass, acids (ex. acetate and butyrate), and alcohols (ex. ethanol and butanol) – all of which derive from acetyl-CoA. The Wood-Ljungdahl pathway is also the most energetically favorable carbon fixation pathway, but its use is confined to only obligate, anaerobic species [1]. Since the discovery of the Wood-Ljungdahl pathway in the early 1980’s, significant effort has been put into trying to characterize the substrate binding activity of CODH/ACS with the M. thermoacetica protein used as the model in most case studies [2]. However, little progress was made in defining the exact structure of the protein with crystal structures until the 2000’s. Several reasons why CODH/ACS has received so much attention include the fact that it contains highly disputed metalloclusters, its use of biological organometallic intermediates in reactions, and its contribution to reducing environmental pollutants [3] [4].
Similar bacteria which harbor monofunctional CODH enzymes include the autotrophs Rhodosprillum rubrum and Carboxydothermus hydrogenoformans [5]. In addition, a variation on CODH/ACS termed CODH-containing acetyl-CoA decarboxylase/synthase is found in methanogenic archea and decomposes acetyl-CoA to carbon dioxide and methane [6].
PDB codes for the M. thermoacetica CODH/ACS enzyme are: 1MJG [7] , 1OAO [8], 2Z8Y [9], 3I01 [10], and 3I04 [11].
Structure
The CODH/ACS enzyme from M. thermoacetica is an with seven metalloclusters. Each 674 residue carries out CODH activity, while each 729 residue is responsible for ACS activity. From the N-terminus to C-terminus, are as follows: an α-helical domain (residues 1-257) followed by two α/β Rossmann-like domains (residues 262-458 and 463-674). The β subunit has 57% helical and 9% β-sheet character with 31 helices and 15 β-strands. The , two with α+β folds and a third with a helical region (residues 1-154) at the N-terminus of a Rossmann (six-stranded α/β) fold (residues 155-316) which is similar to a portion of the β subunit structure [5] [12]. Overall, the α subunit has 50% helical and 14% β-sheet character with 36 helices and 22 β-strands.
(two B-, two C-, and one D-cluster) are located in the β2 portion of the protein with a B- and C-cluster in each β subunit and the sole D-cluster bridging the two subunits. The B- and D-clusters consist of [Fe4S4] centers that serve to transfer electrons. As the active site in each β subunit where CODH activity occurs, the with the unique Fe coordinated by a bridging sulfur ion, His283, and Cys317 [13]. The B- and D-clusters are positioned in relation to the α-helical domain of the β subunit, while the C-clusters are aligned through interactions with both Rossmann-like domains [12]. A ligand binding study with the native CODH/ACS structure and with cyanide used as competitive inhibitor for CO showed that H2O/OH- binds the C-cluster at the unique Fe and CO binds the C-cluster at the Ni ion [2].
The remaining two metalloclusters (both A-clusters) are located in the two α subunits of the CODH/ACS protein. As the active site of ACS activity in each α subunit, the A-clusters are composed of a [Fe4S4] center bridged to a binuclear site. There is some debate regarding the metal ions present in the binuclear site; either [5] or one A-cluster is while the other is [13]. The [Fe4S4] center is coordinated by Cys506, Cys509, Cys518, and Cys528 with Cys509 also forming the bridge between the [Fe4S4] and the proximal metal ion. Furthermore, the distal metal ion is coordinated by Cys595, Gly596, and Cys597 with Cys595 and Cys597 also connecting the distal and the proximal metal ions. Only the [Fe4S4]-Ni-Ni A-cluster is in an open conformation, the other two site types with Cu or Zn as the proximal ion are in closed form.
Another characteristic feature of CODH/ACS is a 138Å long that connects the C- and A-clusters. The cavity network formed by the hydrophobic tunnel is an S-shaped pore from C-cluster to C-cluster with two long branches that lead from the “S” to each A-cluster. As the tunnel runs along nearly the full length of the protein, it enables the intramolecular diffusion of CO from the CODH active sites to the ACS active sites [5].
X-ray crystallography methods were used to determine the structure of CODH/ACS from M. thermoacetica. Crystals were formed by means of sitting drop vapor diffusion. Both molecular replacement (using the CODH structure of R. rubrum [5] and C. hydrogenoformans [13]) and multiple-wavelength anomalous dispersion (MAD) methods were used to solve the structure’s diffraction pattern. Resolution of the structures at the highest resolution shell ranges from 2.5Å to 1.9Å.
Mechanism of Action
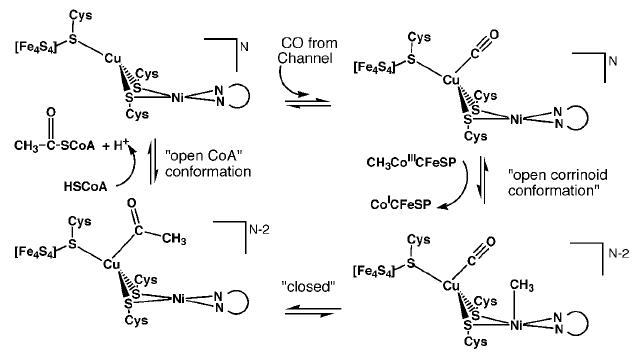 Proposed mechanism 1 for ACS activity with Cu-Ni ions in the binuclear site of the A-cluster. [5]β subunit reactions
C-cluster (CODH activity): CO2 + 2H+ + 2e- ↔ CO + H2O
B- and D-clusters: electron transfer
α subunit reactions
A-cluster (ACS activity): CH3-Co(III)-CFeSP + CO + HSCoA ↔ CH3-CO-SCoA + Co(I)-CFeSP + H+
There are two proposed mechanisms for the catalytic action of the A-cluster: mechanism 1 uses Cu-Ni ions in the binuclear site while mechanism 2 uses Ni-Ni ions.
Mechanism 1 suggests that CO created at the C-cluster travels through the hydrophobic tunnel and binds to Cu. Next, the A-cluster switches to an open corrinoid conformation so that the methyl group may be transferred from the corrinoid iron-sulfur protein to the distal Ni ion of the active site. The cluster reverts back to a solvent protected, closed form to generate the acetyl intermediate, and then reopens to allow HSCoA to bind. HSCoA likely binds in the large cavity between the three domains of the α subunit where six Arg residues and Trp418 coincide. Finally, HSCoA is deprotonated and acetylated to form acetyl-CoA [5].
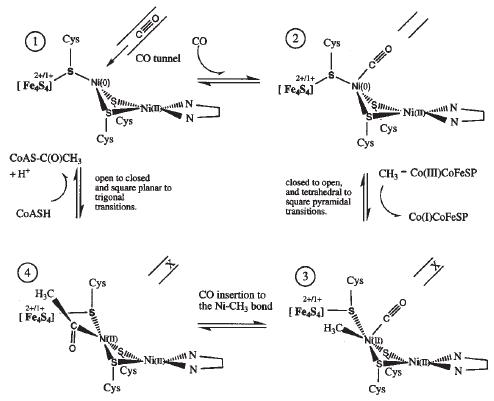 Proposed mechanism 2 for ACS activity with Ni-Ni ions in the binuclear site of the A-cluster. [13]
Mechanism 2 proposes that CO binds to the proximal Ni ion after exiting the tunnel. The A-cluster then changes from the closed to the open conformation which closes the hydrophobic CO tunnel and provides a site for methyl group binding on the proximal Ni as it transitions from Ni(0) to Ni(II). Next, the bound CO inserts into the Ni-CH3 bond to produce an acetyl intermediate. Lastly, deprotonated CoA-S- attacks the carbonyl carbon of the acetyl group to produce acetyl-CoA and the proximal Ni is reduced back to Ni(0) [13]. Note that this mechanism differs from the former by binding both CO and CH3 to the proximal metal ion and also the A-cluster stays open between methylation and CoA acetylation.
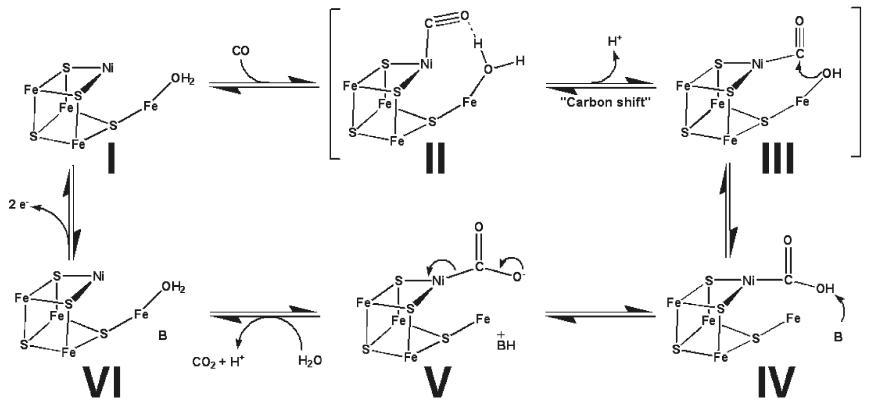 Proposed mechanism for CODH activity in the C-cluster. [2]In the proposed catalytic action of the C-cluster, CO first binds the Ni ion followed by deprotonation of the Fe bound water to yield a reactive hydroxide which promotes nucleophilic attack of CO by the hydroxide. Next, the Ni-COOH intermediate is deprotonated to Ni-COO- which allows release of CO2 as the C-cluster is reduced to a new redox state. Then, electrons are shuttled between the B- and D-clusters to reoxidize the C-cluster to its original redox state [2]. This catalytic cycle is of course operated in reverse to reduce CO2 to CO and allow subsequent production of acetyl-CoA .
Possible Applications
Since CODH/ACS consumes CO2 it plays a direct part in the reduction of greenhouse gases by converting CO2 into an intermediate which can transformed into more desirable products such as alcohols and acids. A novel application in which CODH/ACS plays a role is the production of bioethanol from renewable natural resources such as wood, grasses, and agricultural residues (ex. corn stover). In this process, the biomass undergoes gasification to yield CO, CO2, and H2. The gases are subsequently fed to bacterial reactor systems containing organisms which utilize the Wood-Ljungdahl pathway and thus produce ethanol. The main goal is to produce a renewable energy source that may be used as an alternative to petroleum. However, at this time, the flux of carbon through the Wood-Ljungdahl pathway does not tend to favor ethanol production so much work remains to be done in this area. On the other hand, the Wood-Ljungdahl pathway does produce significant levels of acetic acid, and to this extent, the pathway is considered a biological equivalent to the Monsanto process for industrial acetic acid production [1]. Roughly 10% of the total biological acetic acid production is attributable to anaerobic, autotrophic bacteria which possess CODH/ACS enzymes.
References
- ↑ 1.0 1.1 Berg IA, Kockelkorn D, Ramos-Vera WH, Say RF, Zarzycki J, Hugler M, Alber BE, Fuchs G. Autotrophic carbon fixation in archaea. Nat Rev Microbiol. 2010 Jun;8(6):447-60. Epub 2010 May 10. PMID:20453874 doi:10.1038/nrmicro2365
- ↑ 2.0 2.1 2.2 2.3 Kung Y, Doukov TI, Seravalli J, Ragsdale SW, Drennan CL. Crystallographic snapshots of cyanide- and water-bound C-clusters from bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Biochemistry. 2009 Jul 7. PMID:19583207 doi:10.1021/bi900574h
- ↑ Lindahl PA. The Ni-containing carbon monoxide dehydrogenase family: light at the end of the tunnel? Biochemistry. 2002 Feb 19;41(7):2097-105. PMID:11841199
- ↑ Ragsdale SW, Kumar M. Nickel-Containing Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase(,). Chem Rev. 1996 Nov 7;96(7):2515-2540. PMID:11848835
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Doukov TI, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL. A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Science. 2002 Oct 18;298(5593):567-72. PMID:12386327 doi:10.1126/science.1075843
- ↑ Gong W, Hao B, Wei Z, Ferguson DJ Jr, Tallant T, Krzycki JA, Chan MK. Structure of the alpha2epsilon2 Ni-dependent CO dehydrogenase component of the Methanosarcina barkeri acetyl-CoA decarbonylase/synthase complex. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9558-63. Epub 2008 Jul 9. PMID:18621675
- ↑ 1MJG. DOI:10.2210/pdb1mjg/pdb
- ↑ 1OAO. DOI:10.2210/pdb1oao/pdb
- ↑ 2Z8Y. DOI:10.2210/pdb2z8y/pdb
- ↑ 3I01. DOI:10.2210/pdb3i01/pdb
- ↑ 3I04. DOI:10.2210/pdb3i04/pdb
- ↑ 12.0 12.1 Doukov TI, Blasiak LC, Seravalli J, Ragsdale SW, Drennan CL. Xenon in and at the End of the Tunnel of Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase(,). Biochemistry. 2008 Mar 18;47(11):3474-83. Epub 2008 Feb 23. PMID:18293927 doi:10.1021/bi702386t
- ↑ 13.0 13.1 13.2 13.3 13.4 Darnault C, Volbeda A, Kim EJ, Legrand P, Vernede X, Lindahl PA, Fontecilla-Camps JC. Ni-Zn-[Fe4-S4] and Ni-Ni-[Fe4-S4] clusters in closed and open subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase. Nat Struct Biol. 2003 Apr;10(4):271-9. PMID:12627225 doi:10.1038/nsb912
--Rachel Slivka
|



