Image:EGFRs Mechanism.png
From Proteopedia
Size of this preview: 800 × 365 pixels
Full resolution (1363 × 622 pixel, file size: 38 KB, MIME type: image/png)
Summary
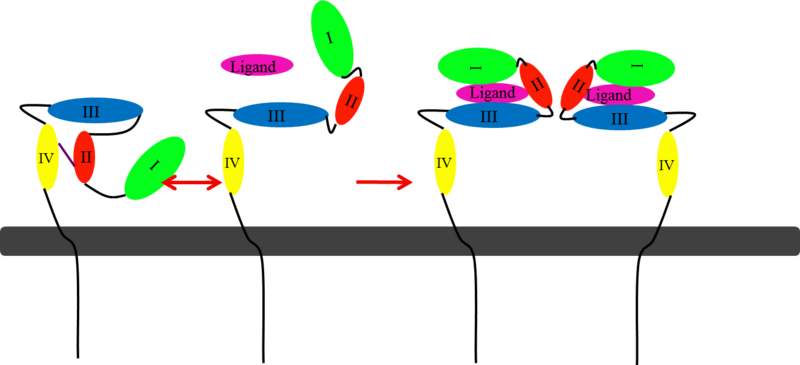
This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction (purple line) with sub-domain IV (yellow). This conformation allows sub-domain II to be hidden and unavailable for dimerization. B) The interaction between sub-domain II and sub-domain IV can be temporarily broken allowing for the receptor to become more available for ligand-binding. C) Upon ligand-binding, if the interaction of sub-domain II and IV is still formed, the interaction between sub-domain II and sub-domain IV is broken and allows sub-domain II to become available for homo- or hetero-dimerization with another receptor from the HER family.
Licensing
| |
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 16:00, 8 November 2012 | Jamie C. Gladfelder (Talk | contribs) | 1363×622 | 38 KB | This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction ( |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
