BamHI
From Proteopedia
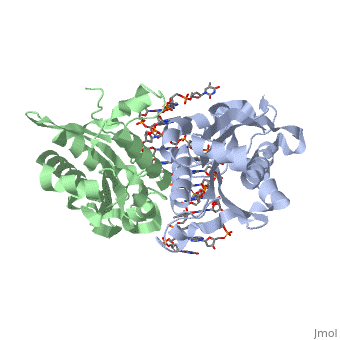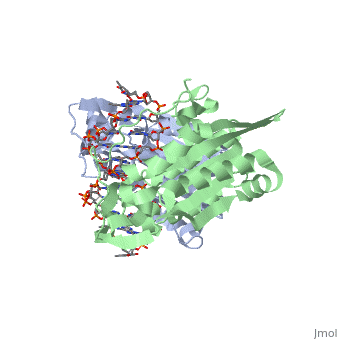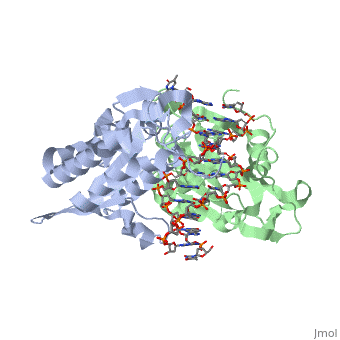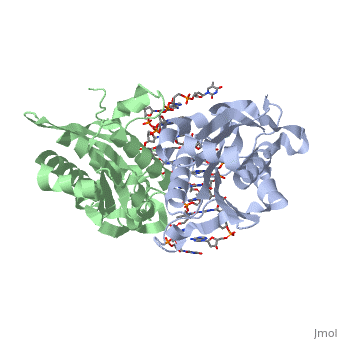
OverviewBamHI is a type II restriction enzyme derived from Bacillus amyloliquefaciens. Like all Type II restriction endonucleases, it is a dimer and the recognition site is palindromic and 6 bases in length. It recognizes the DNA sequence of G’GATCC and leaves an overhang of GATC which is compatible with many other enzymes.[1] The BamHI-DNA complex is a sequence-specific endonucleases-DNA complex. In the complex all hydrogen bonding occurs in the major groove of the recognition site,either through direct or water-mediated hydrogen bonds with the protein, namely on oxygens and nitrogens in the DNA within 3.5 angstroms of the protein. No other DNA sequence could support this degree of complementarity with BamHI.[2] There are five crystal structures of BamHI in the Protein Data Bank. These include BamHI bound to a non-specific DNA, BamHI complex with DNA and calcium ions (pre-reactive complex), BamHI complex with DNA and manganese ions (post-reactive complex), BamHI complex with DNA, and BamHI phased at 1.95 angstroms resolution by MAD analysis.[3] See also Endonuclease. See also BamHI (Hebrew). StructureBamHI consists of 6 alpha helices and 6 beta sheets, surprisingly the protein sequence lacks similarity and has no recurring structural motifs analogous to the helix-turn-helix or the zinc finger of transcription factors.[3] The beta sheet form a curved plane with only two sheets forming hydrogen bonds with specific DNA. BamHI is a dimer of 213 amino acid subunits. The structure of the protein allows hydrogen bonding to occur in the manner seen in this scene. Active Sites and Catalytic Mechanism to Specific DNAType II restriction enzymes require only Mg2+ as a cofactor to catalyze the hydrolysis of DNA phosphodiesters, leaving free 5’ phosphate and 3’ hydroxyl groups. The reaction is considered to proceed by an inline displacement of the 3’ leaving group, in which an activated water molecule acts as the attacking nucleophile. The active site for BamHI are residues Asp94, Glu111, and Glu113. These can be spatially aligned with residues in EcoRI, EcoRV and PvuII. Several mechanisms have been proposed for the way these enzymes might activate a water molecule for nucleophilic attack. It is suggested there is a general base mechanism in which the acidic residues Asp94 and Glu111 coordinate Mg2+ at the active site while Glu113 acts as a general base to deprotonate the attacking water molecule. A separate mechanism has been suggested in which one of the three water molecules located centrally at the active site within hydrogen-bonding distances from the carboxylate groups of Glu111 and Glu113 acts as a nucleophile and attacks the phosphate group of DNA.[4] Active Sites and Catalytic Mechanism for Binding to Nonspecific DNAThe problem of specific versus nonspecific DNA selection is particularly acute for bacterial restriction enzymes. For every BamHI cognate DNA site in the Bacillus amyloliquefaciens H genome, there are 18 sites that differ by only a single base pair. Only the cognate sites are protected from cleavage by methylation produced by the partner methylase enzyme. The stringent specificity for a bacterial restriction enzyme can reduce their cleavage activity by a million-fold with a change in just one base pair. There is an exception in which BamHI binds to a noncognate DNA sequence GAATCC which differs by only one base pair from the cognate G’GATCC sequence. The structure reveals the enzyme is a distinct conformation that is incompetent for cleavage but competent for sliding. The central problem faced by DNA binding proteins is how to select the correct DNA sequence from the sea of nonspecific sequences in a cell. The problem is particularly acute for bacterial restriction enzymes because cleavage at an incorrect DNA site could be lethal. Despite only a single base pair change in the recognition sequence, the enzyme adopts an open configuration that is on the pathway between free and specifically bound forms of the enzyme. Surprisingly, the DNA drops out of the binding cleft with a total loss of base-specific and backbone contacts. Taken together, the structure provides a remarkable snapshot of an enzyme poised for linear diffusion (rather than cleavage) along the DNA. [1] The active sites for BamHI binding to GAATCC are the same as those binding to specific DNA; Asp94, Glu111, and Glu113, but it can be seen that they interact with the ends of the DNA complex rather than hydrogen bonding from the outside as seen in specific DNA binding for hydrolysis. Hydrogen bonding between the protein, DNA, as well as water molecules which are aligned due to the open structure of the protein. There are much more hydrogen bonding interactions through the water molecules in this open structure. |
| |||||||||||
3D structures of BamHI
Updated on 17-January-2022
1bhm, 3bam, 2bam, 1esg – BaBAM + DNA – Bacillus amyloliquefaciens
1bam – BaBAM
References
- ↑ 1.0 1.1 Viadiu H, Aggarwal AK, Structure of BamHI bound to nonspecific DNA: a model for DNA sliding., Mol Cell. 2000 May;5(5):889-95. Print.
- ↑ Voet, Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry Life at the Molecular Level. New York: John Wiley & Sons, 2008. p. 578-579. Print.
- ↑ 3.0 3.1 PDBe., European Molecular Biology Laboratory. 2011. Webpage.
- ↑ Newman, Matthew, Structure of BamHI Endonuclease Bound to DNA: Partial Folding and Unfolding on DNA Binding., Science. 1995 Aug;4(269):656-63. Print.
Proteopedia Page Contributors and Editors (what is this?)
Shane Michael Evans, Michal Harel, Alexander Berchansky, Ann Taylor