Chemical communication in arthropods
From Proteopedia
Contents |
The molecular basis of chemical communication
The sense of smell, Olfaction is a primary sense in nature. It plays a significant role in behaviors which are crucial for the organism survival: food searching, host and mating selection, and avoiding predators and pathogens [3].
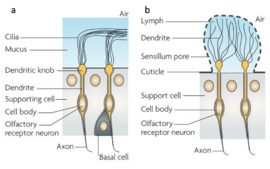
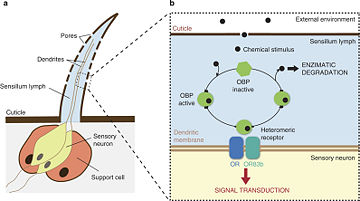
In both arthropods and vertebrates the detection of volatiles is completed by a complicated process which is mediated by soluble as well as transmembrane proteins [4]. It should be mentioned that the detection of pheromones is also vital to microorganisms, as it regulates gene expression in what is termed “quorum sensing”. In arthropods, most of what is known on chemosensory communication is based on research studies in insects. The process begins when a volatile (mostly a small hydrophobic molecule) enters the chemosensilla lymph of an insect, or the mucus of a vertebrate in the nasal cavity (fig 1). Both media are abundant in soluble proteins which bind to the hydrophobic molecules, solubilize and carry the molecule to the chemoreceptors on the dendritic membrane of the olfactory receptor neuron [3][5].The chemical signal is thereby translated into an electrical signal which can cause an immediate response, or further processed with other signals in the insect's mushroom bodies or vertebrate's brain (fig 2)[6][7].
What are the differences and similarities between Arthropods and Vertebrates?
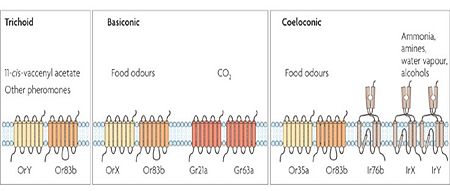
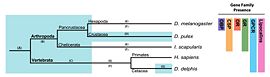
Though functionally similar, receptors as well as soluble proteins are structurally and genetically unrelated in insects and vertebrates (see fig 3 for the putative evolution of proteins involved in chemosensory system).
- Receptors
- Soluble proteins
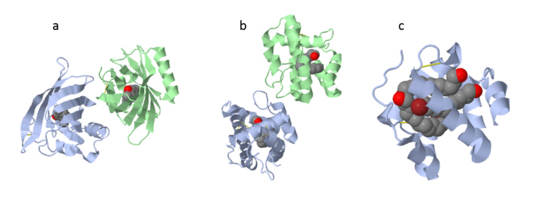
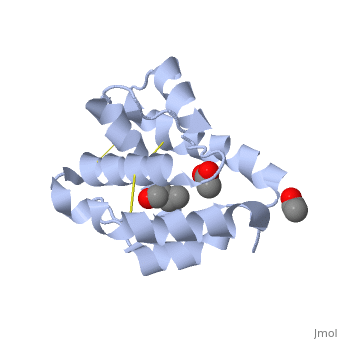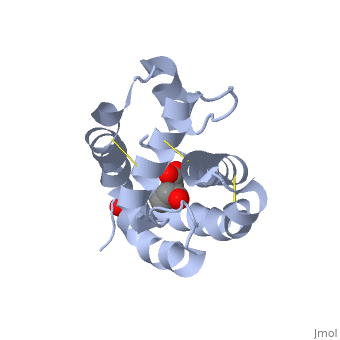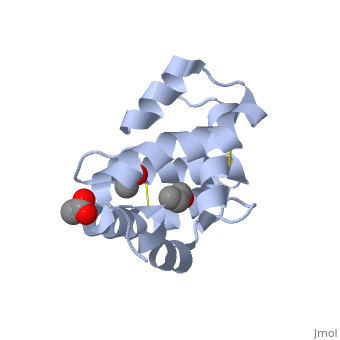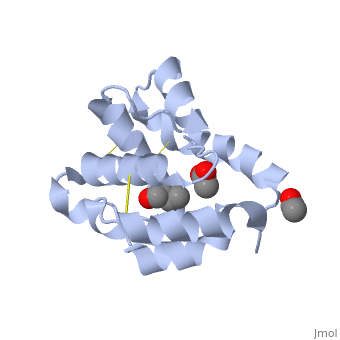
These proteins which are concentrated in the sensillar lymph, solubilize and carry the volatile molecules to the receptor. There are two main known types of soluble proteins that are involved in arthropods' chemical communication: Odorant binding proteins –OBPs,Chemosensory protein-CSP (fig 5). Though bearing the same name and participating in the same function, OBP of vertebrates and arthropods are two distinct families with completely different structure and origin[4]. Arthropods' OBP are composed of alpha helices, while vertebrates' OBP belong to the Lipocalins super family and have a beta-barrel structure (for structure comparison, see table 1 and fig 5). Recently, another family of proteins has been suggested to play a role in ant chemical communication, Niemann-Pick type C2 protein-NPC2 [10].
Types of Soluble proteins in arthropods
| |||||||||||
See also
- Odorant_binding_protein_3D_structures
- For comprehensive explanation about quorum sensing please turn to Fuqua et al. (2001) [16].
- for more information about the protein-ligand interaction, you may go to Odorant binding protein.
References
- ↑ Sanchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity (Edinb). 2009 Sep;103(3):208-16. doi: 10.1038/hdy.2009.55. Epub 2009 May, 13. PMID:19436326 doi:http://dx.doi.org/10.1038/hdy.2009.55
- ↑ Vieira FG, Rozas J. Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol. 2011;3:476-90. doi: 10.1093/gbe/evr033. Epub 2011 Apr 28. PMID:21527792 doi:http://dx.doi.org/10.1093/gbe/evr033
- ↑ 3.0 3.1 Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010 Mar;11(3):188-200. doi: 10.1038/nrn2789. Epub 2010 Feb 10. PMID:20145624 doi:http://dx.doi.org/10.1038/nrn2789
- ↑ 4.0 4.1 Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014 Aug 27;5:320. doi: 10.3389/fphys.2014.00320. eCollection, 2014. PMID:25221516 doi:http://dx.doi.org/10.3389/fphys.2014.00320
- ↑ 5.0 5.1 Vogt RG (2005) Molecular basis of pheromone detection in insects. Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology, eds Gilbert LI, Iatro K, Gills S (Elsevier, London), Vol 3, pp 753–804.
- ↑ Wicher D. Functional and evolutionary aspects of chemoreceptors. Front Cell Neurosci. 2012 Oct 26;6:48. doi: 10.3389/fncel.2012.00048. eCollection, 2012. PMID:23112762 doi:http://dx.doi.org/10.3389/fncel.2012.00048
- ↑ Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373-91. doi: 10.1146/annurev-ento-120811-153635. Epub, 2012 Sep 27. PMID:23020622 doi:http://dx.doi.org/10.1146/annurev-ento-120811-153635
- ↑ doi: https://dx.doi.org/10.1016/S0167-4838(00)00167-9
- ↑ Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008 Apr 24;452(7190):1007-11. doi: 10.1038/nature06861. Epub 2008 Apr, 13. PMID:18408711 doi:http://dx.doi.org/10.1038/nature06861
- ↑ 10.0 10.1 Ishida Y, Tsuchiya W, Fujii T, Fujimoto Z, Miyazawa M, Ishibashi J, Matsuyama S, Ishikawa Y, Yamazaki T. Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc Natl Acad Sci U S A. 2014 Mar 11;111(10):3847-52. doi:, 10.1073/pnas.1323928111. Epub 2014 Feb 24. PMID:24567405 doi:http://dx.doi.org/10.1073/pnas.1323928111
- ↑ Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006 Aug 23;26(34):8727-33. PMID:16928861 doi:10.1523/JNEUROSCI.0876-06.2006
- ↑ doi: https://dx.doi.org/m10.1016/j.cell.2008.04.046
- ↑ Campanacci V, Lartigue A, Hallberg BM, Jones TA, Giudici-Orticoni MT, Tegoni M, Cambillau C. Moth chemosensory protein exhibits drastic conformational changes and cooperativity on ligand binding. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5069-74. Epub 2003 Apr 15. PMID:12697900 doi:10.1073/pnas.0836654100
- ↑ Vanier MT, Millat G. Structure and function of the NPC2 protein. Biochim Biophys Acta. 2004 Oct 11;1685(1-3):14-21. PMID:15465422 doi:http://dx.doi.org/10.1016/j.bbalip.2004.08.007
- ↑ doi: https://dx.doi.org/10.1074/jbc.M703848200.STRUCTURAL
- ↑ Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439-68. PMID:11700290 doi:http://dx.doi.org/10.1146/annurev.genet.35.102401.090913