Group:MUZIC:Myopalladin
From Proteopedia

Contents |
Introduction
Myopalladin (UniProt ID: Q86TC9 [1]) is a 145 kDa protein specific of striated muscles that was identified in a yeast two hybrid screen where the SH3 domain of nebulin (UniProt ID: P20929 [2],[3]) was used as a bait. [1] It belongs to the family of Actin-Associated Scaffolds, which includes myotilin, palladin, and myopalladin, all three being binding partners of α-actinin (for a review, see [2]).
Sequence Annotation
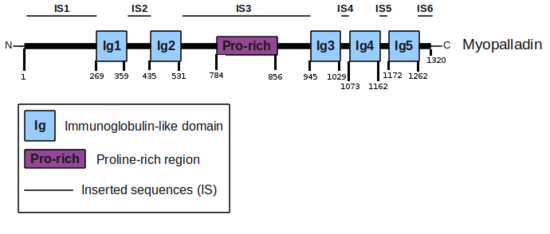
The myopalladin protein is encoded by the MYPN gene (NM_032578). At the protein level, myopalladin comprises 1320 amino-acids organized into 5 domains predicted to be Immunoglobulin-like domains (Ig). These Ig domains are separated by inserted sequences (IS) for which no structural domains could be predicted. The IS3 is a region of low complexity which includes some proline-rich motifs.
Structures
They are currently no structural data available for myopalladin. The only structural data related to myopalladin are the NMR structures of Ig domain 1 (PDB code 2DM2 [[4]]) and Ig domain 2 (PDB code 2DM3 [[5]]) of palladin (homologous to Ig domains 3 and 4 of myopalladin, respectively).
Function and interactions
Function
Myopalladin is mostly localised at the Z disk and the I band of the sarcomere in both skeletal and cardiac muscle cells, and was also found to be present in the nucleus. It is considered to be an important structural member of the Z/I region of the sarcomere. It is involved in the targeting and anchoring of key sarcomeric components (nebulin and nebulette) to the Z-disk and takes part to the α-actinin-based mesh of the Z-disk. Myopalladin is also thought to be related to the Z-disk signaling through its interaction with CARP, a negative regulator of muscle growth.
Ig domains and their binding partners
- CARP: The N-terminal region of myopalladin, going from the N-terminus of the protein to its domain Ig2, was shown to interact with the full length CARP. [1]
- α-actinin: The C-terminal region of myopalladin, going from the domain Ig3 to the C-terminus of the protein, was shown to interact with the EF-hand region of α-actinin. [1]
Inserted sequences and their binding partners
- Nebulin/Nebulette: The IS3 comprises a Proline-rich region that has been shown to interact with the SH3 domain of nebulin and nebulette. [1] [3]
Pathology
Mutations of the MYPN gene were described in patients suffering from Dilated and Hypertrophic Cardiac Myopathies (DCM and HCM). [4] [5] [6]
Many mutations were found in the α-actinin binding region of myopalladin. They are associated with a major disorganisation of muscle cells structure and sarcomere breakdown due to Z-disk instability. These effects are likely to be triggered by the mislocalisation of myopalladin within the muscle cells . [4] [6] More recently, a mutation leading to the truncation of the myopalladin C-terminus part, including both the α-actinin and nebulin binding regions, was shown to have similar effect at the cellular level. [5] On the other hand, a mutation located in the CARP binding-region of myopalladin (Mypn_ Y20C) prevents translocation of myopalladin to the nucleus and is primarily associated to the altered expression of myopalladin binding partners, including CARP, α -actinin and nebulin. [5] Surprinsingly, the down-regulation of α -actinin and nebulin does not affect the structure of the Z-disks. The main effect of this variant is likely to be due to the down-regulation of CARP and, as a consequence, to the up-regulation of CARP-repressed genes, which leads to the hypertrophic phenotype. [5]
References
- ↑ 1.0 1.1 1.2 1.3 Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001 Apr 16;153(2):413-27. PMID:11309420
- ↑ Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31-58. PMID:16164966 doi:10.1016/S0074-7696(05)46002-7
- ↑ Ma K, Wang K. Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett. 2002 Dec 18;532(3):273-8. PMID:12482578
- ↑ 4.0 4.1 Duboscq-Bidot L, Xu P, Charron P, Neyroud N, Dilanian G, Millaire A, Bors V, Komajda M, Villard E. Mutations in the Z-band protein myopalladin gene and idiopathic dilated cardiomyopathy. Cardiovasc Res. 2008 Jan;77(1):118-25. Epub 2007 Sep 19. PMID:18006477 doi:10.1093/cvr/cvm015
- ↑ 5.0 5.1 5.2 5.3 Purevjav E, Arimura T, Augustin S, Huby AC, Takagi K, Nunoda S, Kearney DL, Taylor MD, Terasaki F, Bos JM, Ommen SR, Shibata H, Takahashi M, Itoh-Satoh M, McKenna WJ, Murphy RT, Labeit S, Yamanaka Y, Machida N, Park JE, Alexander PM, Weintraub RG, Kitaura Y, Ackerman MJ, Kimura A, Towbin JA. Molecular basis for clinical heterogeneity in inherited cardiomyopathies due to myopalladin mutations. Hum Mol Genet. 2012 May 1;21(9):2039-53. doi: 10.1093/hmg/dds022. Epub 2012 Jan, 27. PMID:22286171 doi:10.1093/hmg/dds022
- ↑ 6.0 6.1 Meyer T, Ruppert V, Ackermann S, Richter A, Perrot A, Sperling SR, Posch MG, Maisch B, Pankuweit S. Novel mutations in the sarcomeric protein myopalladin in patients with dilated cardiomyopathy. Eur J Hum Genet. 2012 Aug 15. doi: 10.1038/ejhg.2012.173. PMID:22892539 doi:10.1038/ejhg.2012.173