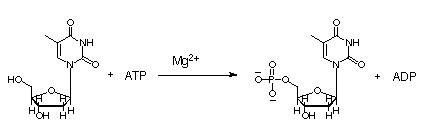
Herpes Simplex Virus Thymidine Kinase
From Proteopedia
| |||||||||
| Herpes simplex virus 1 complex with sulfate ion, 1e2h | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Activity: | Thymidine kinase, with EC number 2.7.1.21 | ||||||||
| Related: | 1kim, 1vtk, 2vtk, 3vtk, 1ki2, 1ki4, 1ki5, 1ki6, 1ki7, 1ki8, 1e2i, 1e2j, 1e2k, 1e2l, 1e2m, 1e2n, 1e2p | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
Thymidine kinases (TKs) catalyze the first phosphorylation step of deoxyribonucleosides in the salvage pathway in DNA synthesis. These enzymes belong to deoxyribonucleotide kinases (dNKs) Humans have two cytosolic kinases (thymidine kinase 1 and deoxycytidine kinase) and two mitochondrial kinases (thymidine kinase 2 and deoxyguanosine kinase). These enzymes have different substrate specificities. Human TK1 differs from the others. Human TK1 phosphorylates has a very narrow specificity; it catalyzes the phosphorylation of only deoxythymidine (dT) and deoxyuridine. It is strictly cell-regulated and has negligible activity in resting cells. Its activity begins in late G1 cells, increases in S-phase and disappear during mitosis.
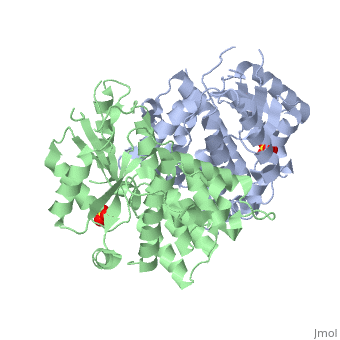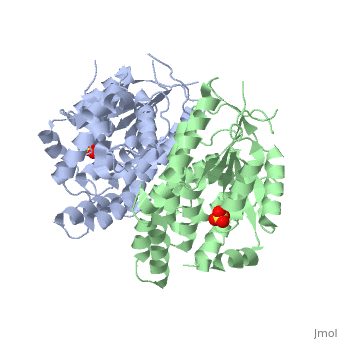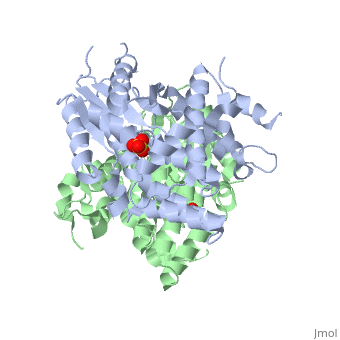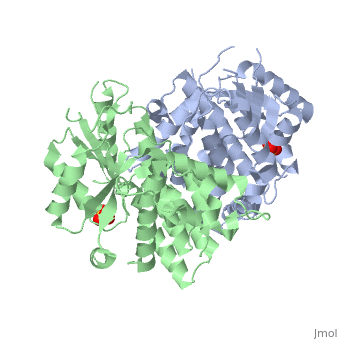
Structure and uses
Herpes simplex virus thymidine kinase (TKHSV1) is a homodimeric α/β- protein comprising of five β-sheets and twelve α-helices per unit. Each unit also has a sulfate ion. It has a two-fold axis. At the interphase are three helices from each monomer where non-polar side chains interact. TKHSV1 is less specific than the human analog; it catalyzes the phosphorylation of pyrimidines, purines and a wide range of ribose analogs. The broad specificity of TKHSV1 has found a lot of therapeutic use in virally directed enzyme prodrug therapy in the treatment of herpes and cancer. The enzyme phosphorylates a non-toxic prodrug to a toxic form that inhibits viral replication. Drugs such as acyclovir for herpes treatment, AZT for HIV treatment and gancyclovir for cancer treatment work in this manner. The drawback with these therapeutic procedures is the poor binding of the enzyme to the drug. A higher dosage is required and this leads to toxicity.
Catalysis and Ligand Binding
At the active center of the enzyme is a glycine-rich P-loop that connects b1 and a1 to form a giant anion hole. The hole accommodates the β-phosphoryl group of ATP, one of its natural substrates. The other natural substrate, deoxythymidine (dT), is located in the pocket formed by a3, a4 and a5. Drugs also insert into this pocket for phosphorylation. ATP binds at the giant anion hole which has a positive charge. This active center is highly conserved in TKs as shown by the consurf analysis.
3D structures of herpes simplex virus thymidine kinase
See Thymidine kinase
Additional Resources
For additional information, see: Viral Infections
References
Christine Wurth, Ulrich Kessler, Joachim Vogt, Georg E. Schulz, Gerd Folkers, Leonardo Scapozza. (2001) Protein Sci. 10: 63-73 Graham Darby, Brendan A. Larder, Moira M. Inglis (1986) J. gen. Virol. 67:753-758 Joachim Vogt, Remo Perozzo, Alex Pautsch, Andrea Prota, Pierre Schelling, Bea Pilger, Gerg Folkers, Leonardo Scapozza, Georg E. Schulz(2000) PROTEINS: Structure, Function and Genetics 41: 545-553