Hoelzer Sandbox
From Proteopedia
Contents |
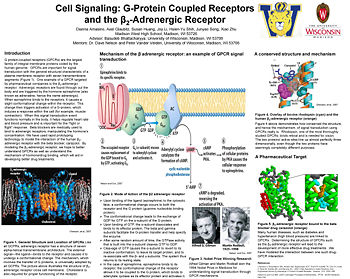
A Physical Model of the β2-Adrenergic Receptor
A SMART Team Molecular Story --- from the Madison West High School 2008 SMART Team
- Students -- Dianna Amasino, Axel Glaubitz, Susan Huang, Joy Li, Hsien-Yu Shih, Junyao Song, Esther Yoon, Xiao Zhu:
- Advisor: Basudeb Bhattacharyya / Assistant: Peter Vander Velden
- Mentors: David Nelson, Ph.D. and Jim Keck, Ph.D., University of Wisconsin-Madison, Madison, WI
|
<swf width="550" height="380">http://myweb.msoe.edu/~hoelzer/Madison West 2007-2008.swf</swf>
Abstract for Our Project
G protein-coupled receptors (GPCRs) are the largest family of integral membrane proteins coded by the human genome. GPCRs are important for signal transduction and share the general structural characteristic of a plasma membrane receptor with seven transmembrane segments. More than 50% of human therapeutics act on a member of the GPCR family of proteins. One example of a GPCR targeted by pharmaceutical companies is the β2-adrenergic receptor. Adrenergic receptors are found throughout the body and are triggered by the hormone epinephrine (also known as adrenaline, hence the name adrenergic). When epinephrine binds to the receptor, it causes a slight conformational change within the receptor. This change then triggers activation of a G-protein --- proteins that bind GTP and are coupled to the receptor on the cytoplasmic side of the receptor --- causing dissociation of the G-protein from the receptor. Through the transfer of GTP, G-protein activates an enzyme that converts ATP into cyclic AMP, which induces a response within the cell (for example, muscle contraction if the receptor is located on a muscle cell). When this signal transduction event functions normally in the body, it helps regulate heart rate and blood pressure and is important for the “fight or flight” response. It is important medically to be able to manipulate these functions in cases of high blood pressure or heart failure through the use of beta blockers, a medicine designed to bind to adrenergic receptors, thus inhibiting the binding of epinephrine, and resulting in a lack of effect of the hormone on the body. We have used rapid prototyping technology to model the interaction of the human β2-adrenergic receptor with the beta blocker, carazolol. The structure is dominated by seven alpha helices and is representative of the structure of GPCRs. By modeling the β2-adrenergic receptor, we hope to better understand GPCRs as well as understand the mechanism of hormone/drug binding, which will aid in developing better drug treatments.
Creating the Physical Model of the β2-adrenergic receptor
|
Virtually any image of a protein that can be created in the computer environment of RP-RasMol, can be converted into a physical model of the protein using rapid prototyping technology. To design our model of the β2-adrenergic receptor, we used the atomic coordinates for this structure as reported in the pdb file 2rh1, from the Ray Stevens laboratory at the Scripps Research Institute.
--- Our model represents .
--- Starting with a cpk-colored, spacefilled representation of the protein, we simplified this image by converting it to an . We colored the seven trans-membrane alpha helices green --- connected by loops that we colored gray.
--- We then displayed four sidechains involved in the binding of a beta-blocker, and colored them blue.
--- The beta-blocker, , was then added in a ball-and-stick format, colored orange.
--- The resolved in this structure, bound to the outside surface of the protein, were added and displayed in a ball-and-stick format, colored red.
--- Finally, of the protein was colored blue, and was colored magenta.
A ply file describing this final structure was exported from RP-RasMol and sent to the MSOE Center for BioMolecular Modeling, where it was constructed from plaster powder, using a color ZCorp 3D printer.
References
1) Rasmussen, S.G.F., Choi, H.-J., Rosenbaum, D.M., Kobilka, T.S., Thian, F.S., Edwards, P.C., Burghammer, M., Ratnala, V.R.P., Sanishvili, R., Fischetti, R.F., Schertler, G.F.X., Weis, W.I. & Kobilka, B.K. (2007). “Crystal structure of the human [bgr]2 adrenergic G-protein-coupled receptor” Nature 450, 383-387.
2) Cherezov, V., Rosenbaum, D.M., Hanson, M.A., Rasmussen, S.G.F., Thian, F.S., Kobilka, T.S., Choi, H.-J., Kuhn, P., Weis, W.I., Kobilka, B.K. & Stevens, R.C. (2007). “High-Resolution Crystal Structure of an Engineered Human 2-Adrenergic G Protein Coupled Receptor” Science 318, 1258-1265.
3) Rosenbaum, D.M., Cherezov, V., Hanson, M.A., Rasmussen, S.G., Thian, F.S., Kobilka, T.S., Choi, H.J., Yao, X.J., Weis, W.I., Stevens, R.C. & Kobilka, B.K. (2007). “GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function” Science 318, 1266-1273.
Our Poster and Presentations
We were able to present our project, poster, and our physical models at the 2008 ASBMB meeting in San Diego, CA. The poster was presented at the Medical College of Wisconsin and at the Scripps Research Institute. During our visit to Scripps, we met Art Olson and David Goodsell --- from the Molecular Graphics Laboratory at TSRI.
MSOE Center for BioMolecular Modeling and SMART Teams
SMART Teams (Students Modeling A Research Topic) is a science outreach program developed by the MSOE Center for BioMolecular Modeling. In this program, teams of high school students work with a local resarch lab to design and build a physical model of a protein that is being investigated by the lab. The goal of the SMART Team program is to introduce students to the real world of science --- as it exists in a local research lab. The development of this program was supported by grants from the NIH-NCRR SEPA program (Science Education Partnership Award) and an HHMI Precollege Science Education Award. For more information about this program, visit the CBM web site at www.rpc.msoe.edu/cbm .