Huan He/Sandbox1
From Proteopedia
Contents |
Interleukin-1 beta(IL-1β)
|
IL-1β along with IL-1α cytokines secreted from local inflammatory cells, are belong to IL-1 family.[1] The former is dominate (90% of Interleukin-1) and the two cytokines could be distinguished by the cells with which they interact. IL-1β precursor(31KDa) need to be activated by protease (convertase or IL-1β converting enzyme[2]) to form IL-1 active species(17KDa)[3]. The active mature IL-1 cytokines could mediate a large range of biological activities such as innate and adaptive immunity as well as bio-components of the acute phase reaction, including the febrile response, secretion of adrenocorticotropic hormone (ACIH), and the synthesis of acute phase proteins.
Human Interleukin-1 Beta
THREE-DIMENSIONAL STRUCTURE OF IL-1β
Quoted from[4]:The molecule resembles a conical barrel with a shallow open face on one end and a closed face on the other. The molecule contains 12 antiparallel 13-strands, where six of these (131, 14, 15, 18, 19, and 1312) constitute an antiparallel 1 barrel. The overall structure of the molecule consists of three similar fragments (Fl, F2, F3), each containing two pairs of 1 strands. Three pairs of 1 strands (one pair from each of the fragments) form the six stranded barrel; the other three pairs cover one end of the barrel, referred to as the "closed end." The amino and carboxy termini are close to each other at the "open end" of the barrel. The molecule has internal pseudo threefold symmetry, with each subunit (Fl, F2, F3) having a 13L13 motif. There are five 1-hairpins in this molecule, two of them in the open end and three at the closed end. 24 hydrophobic side chains line the inner surface of the barrel and both the ends of the barrel have concentrations of exposed polar residues.
are present in the 3D structure. In the picture we could see was colored as purple and was colored as blue, respectively. Most ,which is dark red color, was buried inside of the protein.
TYPE-1 INTERLEUKIN-1 RECEPTOR COMPLEXED WITH INTERLEUKIN-1 BETA
Interleukin-1 receptor complex with ligand and go through the plasma membrane. 3D structure is showing here. Ribbon diagram of s-IL 1R complex to IL-1β. The has approximate dimensions of 97Å×52 Å ×35 Å with one s-IL1R molecule wrapping around the IL-1β molecule with 1:1 ratio. Quoted here[5]: Domain 3 provides a 'lid' which covers most of the top of the IL-1β β-barrel, whereas domains 1 and 2 from a groove which binds to the lower rim of the barrel. Here,Domains 1,2 and 3 of s-IL 1R are colored light, medium and dark blue, respectively. IL-1β is yellow, with site A residues in green and site B residues in red.The structure is oriented so that the carboxy terminus of s-IL 1R and the cell membrane are at the bottom of the picture.
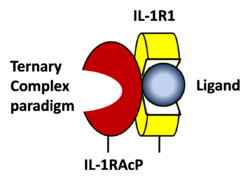
STRUCTURE OF THE INTERLEUKIN-1BETA SIGNALING COMPLEX
3D structure was showed here, and the cartoon (right) showed the formation of the ternary complex paradigm,the primary receptors(IL-1R) first bind their corresponding cytokine ligands(IL-1β) and then engage the accessory receptor(IL-1RAcP) (which is incapable of binding cytokines by itself). The overall ternary architecture of the IL-1β–IL-1RI–IL-1RAcP signaling complex has the cytoplasmic TIR domain necessary for signal transduction. The IL-1RI-IL-1RAcP interface was most hydrogen-bonded and signal was transferred through the highly packed hydrophobic region between receptor accessory and IL-1R liganded with IL-1β[6]
The dimer complex with conserved area was showed when you . The insert are ligands(IL-1β), which are present as conserved structure as well as green ribbon. The two IL-1Rs and two IL-1RAcPs are colored as purple and blue, respectively. The also could be seen.
Clinical significance
Why are people interested in IL-1? Because IL-1 cytokines family are usually over-expressed at tumor sites or inflammatory, these cytokines could be used as bio-markers to help diagnose in advance. Also, since IL-1α, IL-1β and IL-1ra all have the ability to bind to the type 1 IL-1 receptor(IL-1R), and the binding of IL-1α or IL-1β to IL-1R is an early step in IL-1 signal transduction, blocking this interaction may therefore be a useful target for the development of new drugs[5]. For example, in this paper[7], the author therapeutically designed a superior cytokine antagonist(EBI-005, optimized receptor antagonist chimerized with IL-1β and IL-1Ra) for topical ophthalmic use.
Reference
[1] Carl J. March, Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs,Nature,1985,315,641-647.
[2] Cerretti DP,Molecular cloning of the interleukin-1 beta converting enzyme,Science,1992,256,97-100.
[3] Mizutani H, Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase.J Exp Med.,1991,174,821-825.
[4] B Veerapandian, Structure and function of interleukin-1, based on crystallographic and modeling studies,Biophys J.1992,62,112–115.
[5] Guy P. A. Vigers, Crystal structure of the type-I interleukin-1 receptor complexed with interleukin-1β, Nature 1997,386,190-194.
[6] Christoph Thomas, Structure of the activating IL-1 receptor signaling complex,Nature Structural& Molecular Biology,2012,19,455–457.
[7] Hou J, Design of a superior cytokine antagonist for topical ophthalmic use,Proc Natl Acad Sci USA,2013,110,3913-3918.