OspC and Binding
From Proteopedia
OspC and Binding
|
The outer surface protein C (ospC) is a major antigen on the outer surface of the membrane of B. burgdorferi, the spirochete that causes Lyme disease. Although its main functions are largely unknown, ospC’s large degree of polymorphism allows it to be an effective tool in epidemiology and ecology. The different alleles coding for different types of groups of ospC’s are called ospC Major Groups (oMG’s). There are approximately 19 oMG’s, four of which—oMG’s A, B, I, and K—are virulent to humans (Kumaran et al. 2001). Research has found that infection rates of susceptible hosts to virulent oMG’s are higher where genetic variation is low due to bottleneck effects in certain regions of the world, such as the northeastern United States and parts of Europe (Wei-Gang et al. 2002). However, rarer oMG’s also hold a selective advantage in subsequent infections of a host, as the host’s strong secondary response would prevent infection from an oMG to which it has already been exposed (Brisson et al. 2002).
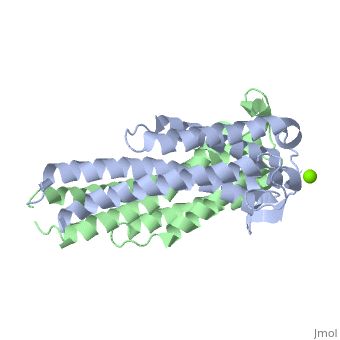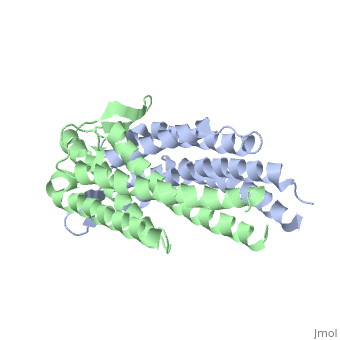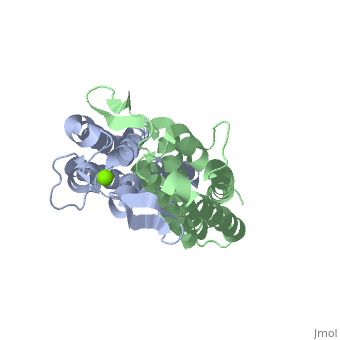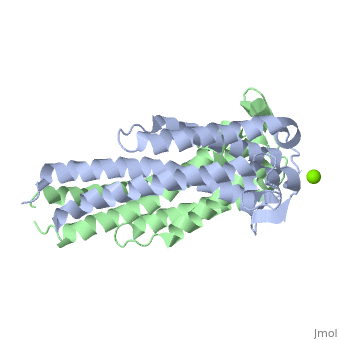
As a kidney-shaped structure with 174 amino acid residues, the ospC is a tetramer composed of two dimers. Most of the structure consists of , which are colored purple in the constructed model of . There is only one on Chain A, marked in yellow.
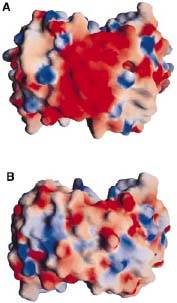
As ospC synthesis is increased upon transfer to a host, possibly due to a temperature increase, there is a possibility of a direct interaction between the ospC and the host (Kumaran et al. 2011). There is a higher density of —marked in red—on one side of each dimer of virulent oMG’s than there is in non-virulent oMG’s (Fig. 1), which suggests a possible binding site for ospC with positively charged host-ligands.
Fig. 1 shows the electrostatic surface potential map of A(HB19) and B(212). HB19 is a virulent strain from oMG A, and 212 is from a non-virulent strain. (Source: Kumaran et al. 2001)
References
- Brisson D, Dykhuizen DE. A modest model explains the distribution and abundance of Borrelia burgdorferi strains. Am J Trop Med Hyg. 2006 Apr;74(4):615-22. PMID:16606995
- Brisson D, Dykhuizen DE. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics. 2004 Oct;168(2):713-22. PMID:15514047 doi:10.1534/genetics.104.028738
- Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, Swaminathan S. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J. 2001 Mar 1;20(5):971-8. PMID:11230121 doi:http://dx.doi.org/10.1093/emboj/20.5.971
- Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics. 2002 Mar;160(3):833-49. PMID:11901105
See also
Proteopedia Page Contributors and Editors (what is this?)
Emily Littman, Alexander Berchansky, Michal Harel, Gayatri Setia