SARS-CoV-2 enzyme Papain-like
From Proteopedia
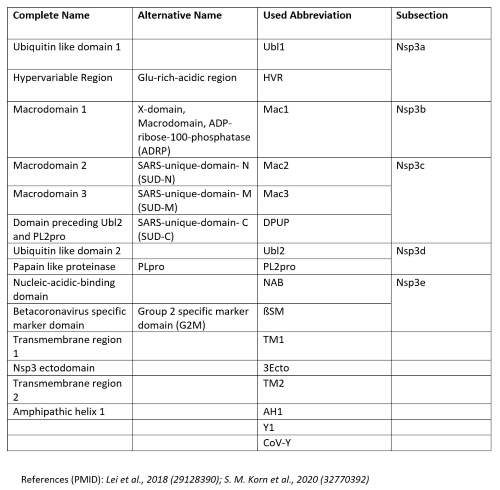
| |||||||||
See also
Coronavirus_Disease 2019 (COVID-19)
SARS-CoV-2_virus_proteins
COVID-19 AlphaFold2 Models
References
- ↑ Modeling of the SARS-COV-2 Genome
- ↑ Zhang C, Zheng W, Huang X, Bell EW, Zhou X, Zhang Y. Protein Structure and Sequence Reanalysis of 2019-nCoV Genome Refutes Snakes as Its Intermediate Host and the Unique Similarity between Its Spike Protein Insertions and HIV-1. J Proteome Res. 2020 Apr 3;19(4):1351-1360. doi: 10.1021/acs.jproteome.0c00129., Epub 2020 Mar 24. PMID:32200634 doi:http://dx.doi.org/10.1021/acs.jproteome.0c00129
- ↑ 3.0 3.1 Yoshimoto FK. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020 Jun;39(3):198-216. doi: 10.1007/s10930-020-09901-4. PMID:32447571 doi:http://dx.doi.org/10.1007/s10930-020-09901-4
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral Res. 2018 Jan;149:58-74. doi: 10.1016/j.antiviral.2017.11.001. Epub, 2017 Nov 8. PMID:29128390 doi:http://dx.doi.org/10.1016/j.antiviral.2017.11.001
- ↑ 5.0 5.1 Wolff G, Limpens RWAL, Zevenhoven-Dobbe JC, Laugks U, Zheng S, de Jong AWM, Koning RI, Agard DA, Grunewald K, Koster AJ, Snijder EJ, Barcena M. A molecular pore spans the double membrane of the coronavirus replication organelle. Science. 2020 Sep 11;369(6509):1395-1398. doi: 10.1126/science.abd3629. Epub 2020, Aug 6. PMID:32763915 doi:http://dx.doi.org/10.1126/science.abd3629
- ↑ Cantini F, Banci L, Altincekic N, Bains JK, Dhamotharan K, Fuks C, Furtig B, Gande SL, Hargittay B, Hengesbach M, Hutchison MT, Korn SM, Kubatova N, Kutz F, Linhard V, Lohr F, Meiser N, Pyper DJ, Qureshi NS, Richter C, Saxena K, Schlundt A, Schwalbe H, Sreeramulu S, Tants JN, Wacker A, Weigand JE, Wohnert J, Tsika AC, Fourkiotis NK, Spyroulias GA. (1)H, (13)C, and (15)N backbone chemical shift assignments of the apo and the ADP-ribose bound forms of the macrodomain of SARS-CoV-2 non-structural protein 3b. Biomol NMR Assign. 2020 Oct;14(2):339-346. doi: 10.1007/s12104-020-09973-4. Epub , 2020 Aug 14. PMID:32803496 doi:http://dx.doi.org/10.1007/s12104-020-09973-4
- ↑ 7.0 7.1 Gallo A, Tsika AC, Fourkiotis NK, Cantini F, Banci L, Sreeramulu S, Schwalbe H, Spyroulias GA. (1)H,(13)C and (15)N chemical shift assignments of the SUD domains of SARS-CoV-2 non-structural protein 3c: "the N-terminal domain-SUD-N". Biomol NMR Assign. 2020 Nov 23. pii: 10.1007/s12104-020-09987-y. doi:, 10.1007/s12104-020-09987-y. PMID:33225414 doi:http://dx.doi.org/10.1007/s12104-020-09987-y
- ↑ 8.0 8.1 8.2 Gao X, Qin B, Chen P, Zhu K, Hou P, Wojdyla JA, Wang M, Cui S. Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharm Sin B. 2020 Sep 2. pii: S2211-3835(20)30698-5. doi:, 10.1016/j.apsb.2020.08.014. PMID:32895623 doi:http://dx.doi.org/10.1016/j.apsb.2020.08.014
- ↑ 9.0 9.1 9.2 9.3 Freitas BT, Durie IA, Murray J, Longo JE, Miller HC, Crich D, Hogan RJ, Tripp RA, Pegan SD. Characterization and Noncovalent Inhibition of the Deubiquitinase and deISGylase Activity of SARS-CoV-2 Papain-Like Protease. ACS Infect Dis. 2020 Aug 14;6(8):2099-2109. doi: 10.1021/acsinfecdis.0c00168., Epub 2020 Jun 4. PMID:32428392 doi:http://dx.doi.org/10.1021/acsinfecdis.0c00168
- ↑ 10.0 10.1 Korn SM, Dhamotharan K, Furtig B, Hengesbach M, Lohr F, Qureshi NS, Richter C, Saxena K, Schwalbe H, Tants JN, Weigand JE, Wohnert J, Schlundt A. (1)H, (13)C, and (15)N backbone chemical shift assignments of the nucleic acid-binding domain of SARS-CoV-2 non-structural protein 3e. Biomol NMR Assign. 2020 Oct;14(2):329-333. doi: 10.1007/s12104-020-09971-6. Epub , 2020 Aug 8. PMID:32770392 doi:http://dx.doi.org/10.1007/s12104-020-09971-6
- ↑ 11.0 11.1 Rut W, Lv Z, Zmudzinski M, Patchett S, Nayak D, Snipas SJ, El Oualid F, Huang TT, Bekes M, Drag M, Olsen SK. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design. Sci Adv. 2020 Oct 16;6(42). pii: 6/42/eabd4596. doi: 10.1126/sciadv.abd4596., Print 2020 Oct. PMID:33067239 doi:http://dx.doi.org/10.1126/sciadv.abd4596
- ↑ 12.0 12.1 12.2 12.3 Klemm T, Ebert G, Calleja DJ, Allison CC, Richardson LW, Bernardini JP, Lu BG, Kuchel NW, Grohmann C, Shibata Y, Gan ZY, Cooney JP, Doerflinger M, Au AE, Blackmore TR, van der Heden van Noort GJ, Geurink PP, Ovaa H, Newman J, Riboldi-Tunnicliffe A, Czabotar PE, Mitchell JP, Feltham R, Lechtenberg BC, Lowes KN, Dewson G, Pellegrini M, Lessene G, Komander D. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020 Aug 26:e106275. doi: 10.15252/embj.2020106275. PMID:32845033 doi:http://dx.doi.org/10.15252/embj.2020106275
- ↑ Baez-Santos YM, St John SE, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub, 2014 Dec 29. PMID:25554382 doi:http://dx.doi.org/10.1016/j.antiviral.2014.12.015
- ↑ Yuen CK, Lam JY, Wong WM, Mak LF, Wang X, Chu H, Cai JP, Jin DY, To KK, Chan JF, Yuen KY, Kok KH. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infect. 2020 Dec;9(1):1418-1428. doi:, 10.1080/22221751.2020.1780953. PMID:32529952 doi:http://dx.doi.org/10.1080/22221751.2020.1780953
Proteopedia Page Contributors and Editors (what is this?)
Lea C. von Soosten, Michal Harel, Joel L. Sussman, Jaime Prilusky