Sandbox20
From Proteopedia
This sandbox is in use for Usyd BCHM3981 until June, 2011. Please do not edit this page.
Contents |
Introduction
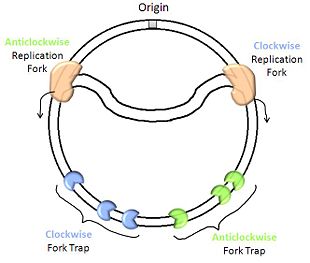
Replication Termination in Escherichia coli and Bacillus subtilis
The replication of chromosomal DNA in most bacterial species occurs through a bidirectional mechanism, whereby two replication forks derived from the same origin of replication travel in opposite directions.[1] This gives rise to the characteristic theta-shaped structure while the nascent DNA loop connecting the replication forks is generated. While the use of two replisomes, one at each fork, accelerates replication, the phase of termination must be carefully coordinated. Specific sequences known as Ter sites lie just beyond the halfway point for each replisome. [2] These halt the advance of replication forks in a direction-specific manner, and thereby form a replication fork trap. As a result, each replisome can traverse only slightly more than half of the DNA before it is arrested. Together, the two sets of Ter sites define the terminus region where replication is terminated and the two forks fuse. [3]
The function of the replication fork trap is enacted by the binding of termination proteins to Ter sites. In E. coli, this is done by termination utilisation substance (Tus), and the functionally corresponding protein in B. subtilis is replication termination protein (RTP). [4] The importance of these replication fork traps is still a matter of debate, as their inactivation does not interfere greatly with cell cycle progression[5], nor does it affect the well-being of daughter cells following cell division. However, the fact that the structurally and sequentially dissimilar Tus and RTP proteins both bind to ill-conserved Ter sites to elicit the same biological function suggests an evolutionary pressure towards a termination mechanism that involves replication fork traps. It has been noted that the majority of genes in the B. subtilis genome have their promoters proximal to the origin of replication. [6] Terminating replication before the replisome reaches the promoters may help the smooth running of transcriptional processes. [7]
During fork fusion, it is proposed that the fork arriving at the permissive face of the replication fork trap displaces the terminator protein and causes the disassembly of the replisome. A combination of helicase, toposiomerase, polymerase and ligase then fill the remaining gaps to make a chromosome dimer with the two full-length chromosomes joined. RecQ, topoisomerase III, single-strand binding protein (SSBP) and DNA polymerase I are said to play a role in this process [8]. Finally, site-specific recombination takes place at the dif site to produce two identical monomeric chromosomes [9].
The Proteins
RTP
In B. subtilis, Ter sites are 30 bp in length with two imperfect inverted 16 bp repeats overlapping at a TAT motif.[10] The upstream portion of the Ter site is called TerA; and the downstream portion, TerB. The difference in sequence between these cause the RTP dimers to bind with different affinities and this generates an capable of halting the progression of the replication fork when the B site is encountered first.[11]
Structure of the RTP Dimer
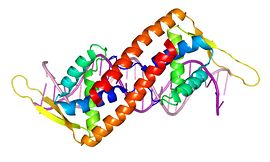
The structure of an RTP monomer bears greatest similarity to the classic winged-helix motif, in which 'wings' project from the loop between the final two β sheets of the compact αβααββ structure.[12] The two major variations from this theme are the absence of a β1 sheet (the corresponding region is instead termed the β1 loop), and the presence of a fourth elongate α-helix at the C-terminus, which facilitates dimerisation.[13] Each of these secondary structural elements are indicated in this .
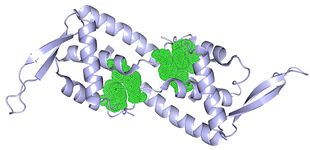
The association of α4 helices into an antiparellel coiled coil leads to dimerisation (right). The conformation is further stabilised by interhelical salt bridges outside this region, as well as an aromatic network that forms a hydrophobic core on the inner surface. [14] The phenylalanine and tryptophan residues that are part of this network are shown in the image (below).
The crystal structure of RTP in its unbound state was determined in 1995. A C110S mutant was then generated to prevent the aggregation of RTP through cysteine oxidation. With a very similar structure and almost no change in dimerisation and DNA-binding capacities, the mutant was the protein of choice for later studies.[15]
DNA Binding
|
When an RTP dimer binds to a TerA or TerB site, the basic residues of the α3 helix are positioned in the major groove, and the β-ribbon rests within the minor groove.[16] Non-specific ionic interactions between the N-terminus and the DNA backbone then stabilise this complex.
It is known with some certainty that the RTP dimer adopts an asymmetric arrangement upon binding of the TerB site. [17] In the C110S mutant complexed with the native TerB sequence, the two subunits interact differently with the DNA bases to produce wing-up and wing-down conformations.[18] These can be distinguished by the angle the α2 helix makes with the α3 helix indicated in this . It is likely that asymmetry is also introduced when the RTP dimer binds to the TerA site, but the crystal structure of this complex has not been solved.
The dissociation constant of the RTP-TerA complex is greater than that of RTP-TerB. This indicates an inherently lower binding affinity of RTP for TerA.[19] However, following the binding of RTP to TerB, a positive cooperative effect facilitates the binding of RTP to TerA.[20] It has been proposed that RTP bends the DNA at the TerB site in a manner that favours RTP binding at TerA. The RTP dimer at TerB may also present a surface for stabilising interactions with the dimer at TerA through its β1 loop and β3 strand.
Replication Termination Activity
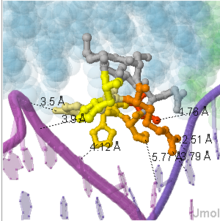
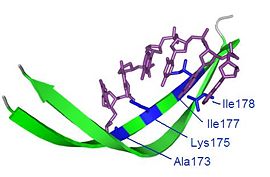
The asymmetric arrangement of the RTP dimer means that certain regions of the protein are accessible from one face only. In particular, Tyr33 makes contact with the replication fork in the wing-down monomer only, as shown in this . Interestingly, the residues around Tyr33 carry some similarity to the residues of the leading face of DnaB helicase, suggesting possible interactions between the two proteins, which may contribute to the suppression of helicase activity [21]. For unknown reasons, both the TerA and TerB sites must be occupied for full replication termination activity. [22]
Index of RTP scenes: [1] , [2] , [3] , [4] .
Tus
|
Tus is the 36 kDa protein responsible for termination of replication in Escherichia coli.[23] The E. coli chromosome contains a set of six polar Ter DNA sequences arranged such that three with the same directionality are located on either half of the chromosome to the form the fork trap.[24] The Ter sites contain a 20 bp consensus element to which a Tus monomer binds. However, while RTP likely stops DnaB helicase activity through direct protein-protein interactions, Tus does so by displacing a cytosine residue to disrupt the normal DNA conformation that is required for helicase activity.[25]
Structure of the Tus-Ter Complex
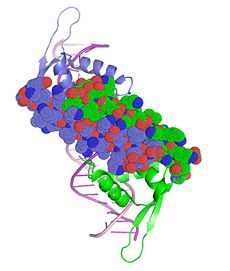
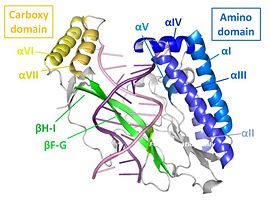
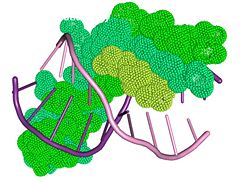
The structure of the Tus protein in complex with TerA revealed a previously undescribed backbone conformation ().[26] With an amino and a carboxy domain, the α-helical regions of each domain are spanned by a central β-sandwich, which makes contact with the DNA duplex. Three helices within the amino domain (αI αII, αIII) form an antiparallel bundle that is aligned parallel to the DNA (blue, left). Another two helices (αIV, αV) clamp the DNA backbone at the non-permissive end. This generates a cytosine-specific pocket with the residues required for anti-helicase activity.[27] The main DNA-binding domain is, however, the exposed side of the double β sheet layer, which provides several base-specific interactions. This lies within the major groove and causes a conformational change in the DNA involving a deepening of the major groove, and an expansion of the minor one (green, left).[28]
DNA Binding
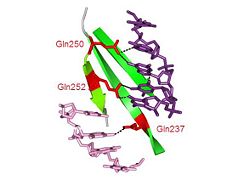
Tus is among the most stable monomeric, sequence-specific, double-stranded DNA-binding proteins.[29] This is due to a combination of three major sets of interactions; base-specific polar interactions within the major groove, non-polar contacts with the carboxy domain, and a phosphate clamp within the amino domain. The three β-sheets which span the major groove of DNA make both base-specific and non-specific bonds, as shown in this . In particular, three glutamine residues on the βJ strand form bidentate hydrogen bonds to bases at the permissive end of the complex (below centre).[30] Interspersed with these residues on the same strand, residues such as isoleucine make Van der Waals and hydrophobic interactions to sugar and base moieties (below right). The distribution of bonds to each strand of the duplex is highly asymmetric, and one strand is largely exposed to the solvent. This contributes to allowing the passage of the fork from one side only. [31]
The phosphate clamp is located at the end of the aIV and aV helices, closest to where the replication fork is stalled. [32] It ensures the protein does not come loose at the critical end and allow helicase activity to occur. It involves five, mostly van der Waals, contacts with the sugar-phosphate backbone.[33]
Replication Termination Activity
The displacement of a nucleotide from the double helix determines the polarity of fork arrest.[34] This cytosine is located at the edge of the non-permissive face, and defines the point at which helicase activity is halted. Upon interaction with Tus, the cyotsine is no longer base-paired, and is instead associated with residues within a well-defined recognition pocket.[35] The specific interactions which stabilise this are shown in this .
Before the structure of the Tus-Ter complex was solved, mutation of Glu49 was shown to eliminate anti-helicase activity without affecting DNA binding.[36] This could not be explained by the original crystal structure as it is not located close enough to make direct contact with the conserved cytosine.[37] However, a more recent structure revealed the reason to be disruption of a water-mediated hydrogen bond between the Glu49 and the adenine residue next to the cytosine.[38], as shown in this .
Index of Tus scenes: [1] , [2] , [3] , [4] , [5] .