---
[1]
Introduction
[1]
Structure
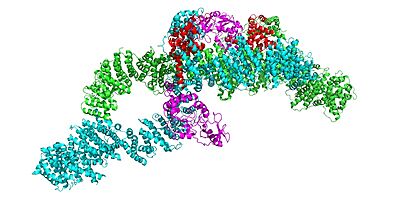
Neurofibromin is a with two identical chains (depicted as lime and cyan).
Conformation: Open
Conformation: Closed
In the overall closed and inactive conformation of the neurofibromin protein, both sets of the GRD and Sec14-PH domains are rotated in a way that they are inaccessible and inactive. The closed conformation has both protomers/chains in the closed structure, whereas the open conformation has one closed and one open protomer. You can see that in the closed conformation, Ras binding by the GRD domain is sterically hindered and there is no room for association with the Ras protein.
The closed conformation has the GRD and Sec14-PH domains oriented in a way that the amino acids C1032, H1558, and H1576 are in close proximity to each other to form a transition metal-binding site with zinc. The fourth coordination partner in this is water.
Domains
The of Neurofibromin, specifically the arginine finger (R1276), binds to the Ras + GTP complex.
Key Players
This is because an (R1276) present in the GRD is critical for Ras binding and is only accessible when the GRD and Sec14-PH domains are rotated in such a way that there is no steric hindrance from the surrounding dimer chains.
Closed conformation is stabilized by one cysteine and two histidines that are coordinated with transition metal-binding sites with zinc.
Function
Ras Control
Ras is still promoting cell proliferation in this closed conformation because Neurofibromin is unable to hydrolyze Ras and inactivate it. In this open conformation, the Ras is not sterically hindered and the Arginine finger is accessible for Ras binding, thus allowing Neurofibromin to down-regulate Ras.
Mutated
Diseases
[2]