Sandbox Reserved 197
From Proteopedia
| This Sandbox is Reserved from Feb 02, 2011, through Jul 31, 2011 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 191 through Sandbox Reserved 200. |
To get started:
More help: Help:Editing |
Contents |
Introduction
Ribonuclease A is an enzyme found in the pancreas that is involved in catalyzing RNA degradation [1]. The kidney-shaped structure of RNase A has been determined through crystallography [2] and Fast Atom Bombardment Mass Spectrometry [3]. FABMS is performed by mixing the material to be analyzed (RNase A) with a non-volatile environment called a matrix and is then bombarded by high energy molecules within a vacuum. Bombardment causes the molecules to ionize so they can be detected by mass spectrometry. Through this technique it has been found that RNase A is composed of four anti-parallel β-sheets and three α-helixes. Presence of four disulfide bonds and two cis proline residues greatly effects the structure and folding kinetics of RNase A [4]. When RNase A undergoes reductive denaturation, it spontaneously folds back on itself to form the same structure. This observation of ribonuclease folding helped Christian Anfinsen win the Nobel Prize in 1972 for his work on protein folding [5].
Protein Folding
Interatomic interactions, delegated by the amino acid sequence, are responsible for formation of a protein's 3D structure [6]. Several of these interactions have been identified by the use of site directed mutagenesis to wildtype RNase A and subsequent comparison of the crystal structure to the wildtype. Although RNase A has 105 possible disulfide bond pairings, only one set of four bonds occurs. This unique observation leads to the "thermodynamic hypothesis", that a protein's native state is determined by the thermodynamic favorability of the whole system; thus the tertiary structure must be predetermined by intramolecular interactions within the amino acid sequence. Since thermodynamic stability of a protein is affected by the environment's temperature, pH, and ionic strength, among other factors, the protein structure can only exist under physiological conditions. Today, the correlation between the amino acid sequence and the tertiary structure of RNase A continues to serve as a model for protein folding. Among the most important attributes of this model are noncovalent interactions, proline conformation, and disulfide bonding.
|
Proline Conformation
The presence of cis [7]proline residues plays a large role in protein folding. In nature, most amino acids reside in a trans conformation, but due to their cyclic structure, prolines are more stable in the cis conformation than any other amino acid. RNase A contains four proline residues, two reside in the cis conformation and two in the trans conformation. The importance of these conformations are demonstrated based on the structure of RNase A variants with several mutations to the wild type amino acid sequence. Located in an outer of RNase A, the peptide group of RNase A in its native state is found in the cis conformation. When proline was mutated to alanine, , a cis conformation still forms at position 93 which is an energetically unfavorable conformation for an alanine residue [8]. Upon unfolding, Tyr92-Ala93 undergoes isomerization to form its more favorable trans conformation demonstrating that the cis conformation is favored by other interactions within the folded protein structure. Although the overall structure of RNase A is not affected by this mutation, the rate of folding greatly decreases upon insertion of the P93A mutation, suggesting an important kinetic contribution of cis prolines to protein folding.
The peptide bond also resides in a cis conformation in its folded structure, but exists in the trans conformation in its unfolded state; therefore, steric restraints imposed by the rest of the protein must be responsible for this cis conformation. Unlike P93A, the insertion of a point mutation causes the peptide bond to adopt a trans conformation and causes a 9.3 Å movement of the loop [9]. The kinetic rate and overall native conformation are not significantly effected by this mutation; however, locally, a rearrangement of the hydrogen-bonding network occurs. Results of this mutation confirm that steric hinderance of the protein can lead to formation of the cis conformation by a proline and is further energetically stabilized by hydrogen bonding, Van der Waals, and electrostatic interactions within the protein.
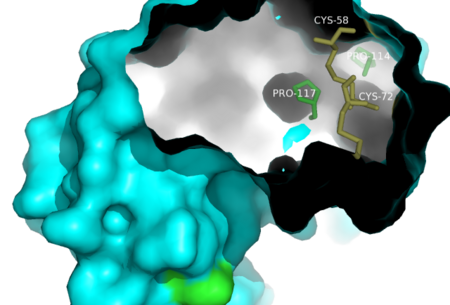
Another important role of proline residues is their involvement in β turns. β turns are 180° turns commonly found in globular proteins to allow for a compact structure by connecting the ends of adjacent antiparallel β sheets [10]. The turn consists of a sequence of four amino acid residues. The carbonyl of the first amino acid hydrogen bonds with the amino group of the fourth amino acid. Proline is involved in β turns because it is small, flexible, and assumes a cis conformation, all attributes that allow for formation of a turn. In RNase A both Pro93 and Pro114 are involved in β turns. Proline residues are important to protein folding because their ability to form a favorable cis conformation allows for thermodynamic favorability of β turn formation. With β turns, amino acids can fold back on themselves allowing the protein to reside in a compact, globular structure.
|
Disulfide Bonds
Another important feature of the folding of RNase A is the presence of four disulfide bonds. These bonds contribute to the thermal stability and the rate of folding of RNase A. The residues involved in these linkages include , , , and . Cys26-Cys84 and Cys58-Cys110 stabilize an interaction between an α-helix and a β-sheet which is the main contributor to the thermodynamic stability of the enzyme. Measurements of protein activity upon removal of disulfide bridges show that the change in enzymatic activity is very small and that not all disulfide bridges are essential for the structure or the reactivity of the protein. However, removal of disulfide bonds does destabilize the hydrophobic core and decreases the rate of folding. RNase A actually has a rate-determining three-disulfide intermediate. An analog of this, , shows RNase A, missing the disulfide bond, Cys40-Cys95, that would normally occur here. In the variant, only 3 disulfide bonds are present, but the overall structure is only changed slightly. The differences occur in residues in close proximity to the location of the missing disulfide bond, and , where there are increased levels of disorder and a destabilized hydrophobic core [11].
Summary
Protein folding is not due to one interaction, but a network of interactions within the protein. When a proline residue or disulfide bond is removed from RNase A, the structural changes are usually confined to the site of mutation and minor structural changes occur within close proximity to the mutation. Although the effects of mutations seem to be localized, mutating proteins greatly effects the stability of the molecule and the rate of folding.
Medical Importance
Protein folding has several medical implications. Diseases such as ALS, Alzheimer's Disease, and Parkinson's Disease can all be traced back to protein folding because proteins can form aberrant aggregates when they do not fold correctly. This abnormality can be toxic to human nerve cells. All proteins contain . The hydrophilic residues lie on the outer part of the protein and the hydrophobic residues bury themselves within the interior of the protein due to the hydrophobic effect [12]. Mistakes made during protein folding may cause a protein to expose and, in turn, cause several proteins to stick together and form a plaque. In the future researchers hope to design drugs that combat mistakes in protein folding [13]. The use of ribonuclease A in protein folding research has been an instrumental feature in designing experiments to determine these "misfolding" snapshots and in developing therapies to prevent protein misfolding.
References
"Christian Anfinsen - Nobel Lecture". Nobelprize.org. 15 Apr 2011. http://nobelprize.org/nobel_prizes/chemistry/laureates/1972/anfinsen-lecture.html
Hogan, Dan. ed. Dysfunctional Protein Dynamics Behind Neurological Disease? ScienceDaily.2 Nov. 2009. www.sciencedaily.com
Lehninger A., Nelson D.N, & Cox M.M. (2008) Lehninger Principles of Biochemistry. W. H. Freeman, fifth edition.
Pearson, M.A., Karplus, P.A., Dodge, R.W., Laity, J.H, and Scheraga, H.A. (1998) Crystal structures of two mutants that have implications for the folding of bovine pancreatic ribonuclease A. Protein Science. 7:1225-1258.
Raines, R.T. (1998) Ribonuclease A. Chem. Rev. 98:1045-1065.
Schultz, D.A., Friedman, A.M., White, M.A., and Fox, R.O. (2005). The crystal structure of the cis-proline to glycine variant (P114G) of ribonuclease A. Protein Sci. 14:2862-2870.
Sela, M. (1957) Reductive cleavage of disulfide bridges in ribonuclease. Science 125(3250):691-692.
External Links
http://en.wikipedia.org/wiki/Ribonuclease_A
http://nobelprize.org/nobel_prizes/chemistry/laureates/1972/anfinsen-lecture.pdf