Sortase enzymes are trans-peptidases found in Gram-positive bacterial species. Their purpose is to covalently link proteins to the cell wall. By recognizing a specific sequence on target proteins, they “sort” which proteins to attach. Different sortases are separated into different classes based on their recognition sequence and specific function. Class A sortases (SrtA) found in Staphylococcus aureus were the first sortase enzyme to be isolated in the lab in 1999 and have become the prototypical sortase [1]. Because surface proteins play such a big role in a pathogen’s virulence, sortases have become an important topic for study [2].
Structure
Sortases usually consist of 200-300 amino acids, with a typical molecular weight between 20-30 kDa. The enzyme possesses a hydrophobic transmembrane α-helix at the N-terminus, with the majority of the protein on the outside of the membrane. They have a typical “sortase-fold” that consists of an uneven β-barrel made up of eight β-strands. The connecting loops form the walls of a groove where the active site rests. It is these loops that are thought to give these enzymes their specificity [1].
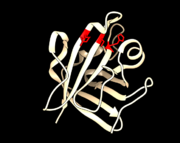
PDB 1T2W Active Site Reisdues Arg 197, Cys 184, and His 120 highlighted
S. aureus SrtA mechanism
In Gram-positive bacteria, SrtA enzymes are almost always present. Their prevalence, combined with them being discovered first, has led to them having the most extensive mechanistic studies of all the sortases. The active site is composed of an Arginine residue (Arg 197), a Cystine residue (Cys 184), and a Histidine residue (His 120). SrtA has a LPXTG recognition sequence that is highly conserved. Mobile loops peripheral to the active site bind to this sequence near the C-terminal. In S. aureus a calcium ion binds behind the substrate binding groove and locks the loops into a binding position. The Arg197 residue hydrogen bonds to the carbonyl on the peptide backbone of the substrate, meanwhile the Cys184 deprotonates into a thiolate ion to perform a nucleophilic attack on the bond between T and G on the substrate. The His120 residue donates a proton to the amine leaving group of the substrate’s C-terminal domain. Then an amine on the pentaglycine crossbridge protein of a peptidoglycan precursor enters the active site and has a proton abstracted by the histidine. This increases the amine’s nucleophilicity enabling a nucleophilic attack on the substrate. This dislocates the substrate from the enzyme and restores the active site, resulting in the two peptides bonded together. This process is entirely driven without spending ATP [1].
Sortase Classes
Class A
Sortase A enzymes, like SrtA, are known as “housekeeping” sortases. They recognize the sequence LPXTG and are nearly universally found in all Gram-positive bacteria. They sort and attach a wide variety of cell surface proteins to the cell wall [1].
Class B
The acquisition of iron plays an important role in the creation of bacterial infections and Class B sortases bind proteins that help sequester iron from the environment. SrtB is a class B sortase found in both S. aureus and Bacillus anthracis. In both organsims they are associated with binding the protein IsdC to the cell wall which binds to heme and utilize it as a source of iron [3]. The sequence recognized by class B sortases is NP(Q/K)(T/S)(N/G/S)(D/A) displaying a wider variety of signals. Class B sortases noticeably attaches proteins to different sites in the cell wall than class A sortases. Another difference between SrtA and SrtB is that SrtB and the iron acquiring IsdC are only expressed under iron deficient conditions [2].
Class C
Pili may extend 0.2-3.0 μm from the cell surface and promote cell adhesion and the formation of biofilms. In Gram-positive bacteria class C sortases are used to construct pili, linking together the pilin subunits via isopeptide bonds and only sometimes connects the pilus to the cell wall itself. The general process is conserved, but there is a greater variety in the structure or number of sortases and accessory factors needed [2]. The recognition sequence for class C sortases is QVPTG [1].
Class D
Class D sortases have so far only been studied in B. anthracis, where it was found to attach BasH and BasI to the cell wall. The class D sortase manages to attach each protein to different structures in the sporulating cell. Deleting the class D sortase reduced the efficiency of sporulation [2]. The recognition sequence for class D sortases is LPNTA [1].
Class E and F
Less is known about class E and F sortases. Class E sortases are believed to be an alternative housekeeping sortase to class A, as the two are never found in the same genome. They recognize the sequence LPXTG. Class F sortases are found in Actinobacteria, but their function is currently unknown [2].
Industrial and Laboratory Applications
Sortases may be very useful in the lab as they can be used to attach a variety of probes to any protein. The probe contains the LPXTG recognisiton sequence for S. aureus SrtA (or LPXTA for Streptococcus pyogenes SrtA) and the target protein acts as the nucleophile in the active site. The reaction can be accomplished in less than three hours [4]. Sortases can also be used for in vitro protein ligation. They can ligate a peptide with the correct motif to an aminoglycine peptide. They can also be used to conjugate synthetic branched peptides, (d)-peptides, and aminoglycine-derivatized small molecules to the C terminus of any recombinant protein[5].

