Sean Swale/Human Thrombin Inhibitor
From Proteopedia
| |||||||
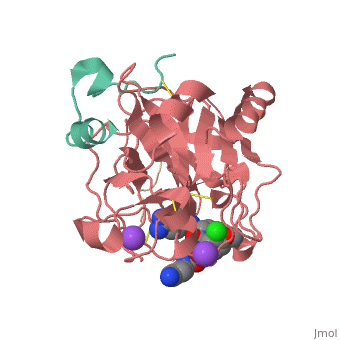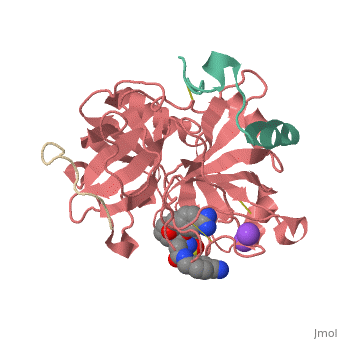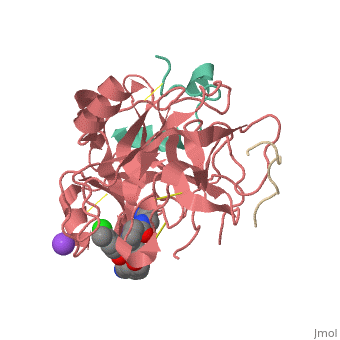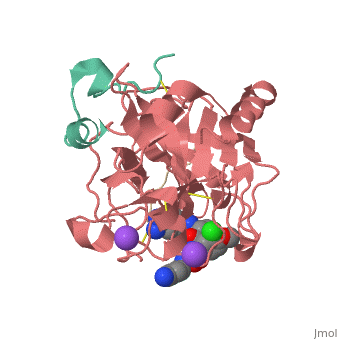
| Human Thrombin, heavy chain (red), light chain (aqua) complex with hirudin (gold), pyrrolidine derivative and Na+ ion 3utu | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||
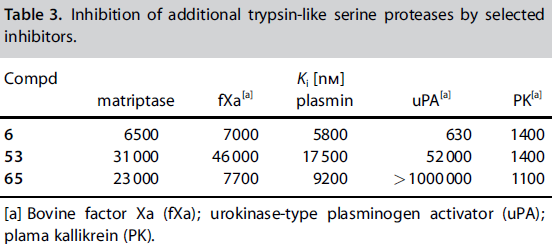
| Non-Standard Residues: | |||||||
| Activity: | Thrombin, with EC number 3.4.21.5 | ||||||
| Related: | 3dt0, 2zfo, 3eqo, 3f68, 3dux, 2ziq | ||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Reasons to develop another Inhibitor
Thrombin is a serine protease that cleaves fibrinogen to allow fibrin to form stringy networks that trap red blood cells to form clots. Thrombin is a serine protease because it cleaves fibrinogen with its serine residue. Thrombin when made by the body is tethered to blood vessels so that it cannot cause clots throughout the body causing strokes and heart attacks. Additionally, thrombin is only activated for a few seconds to limit the clotted area to the injured area[1]. However, when fibrin has accumulated at an injury site, sometimes it will break away and occlude a blood vessel which can cause stroke or heart attack. In order to prevent venous thrombosis, blood clots, after surgery or during dialysis when blood is in contact with artificial devices, Heparin is normally administered. Unfortunately Heparin is neutralized by plasma proteins in the blood, is less efficient for the inhibition of clot bound thrombin, and can trigger an enormous immune response. Antithrombotic drugs like Hirudin will be given if Heparin is not able to be tolerated or if the person receives regular dialysis. Hirudin administration require large doses to maintain proper inhibition of Thrombin which heavily taxes the body. Chemists have been working on designer drugs to increase selectivity and potency. One compound which has shown great selectivity and potency in vitro is .[2]
Phe-Pro-p-amidinobenzylamine
An early experiment to find a better thrombin inhibitor yielded a structure that was able to bond to the P1 and P2 sites of Thrombin effectively. In 1996 Lilly research laboratories created D-Phe-Pro-p-amidinobenzylamine by making improvements on the previous structure D-Phe-Pro-Agmatine. The new compound they created bonded to thrombin 130x more than it did to trypsin (also a serine protease), and it was highly selective of thrombin over fibrinolytic enzymes which break down fibrin clots. The inhibitor bound with Thrombin at rates 26,000x greater than with plasmin,[3], 170,000x greater than with t-Pa, and 400,000x greater than with urokinase.
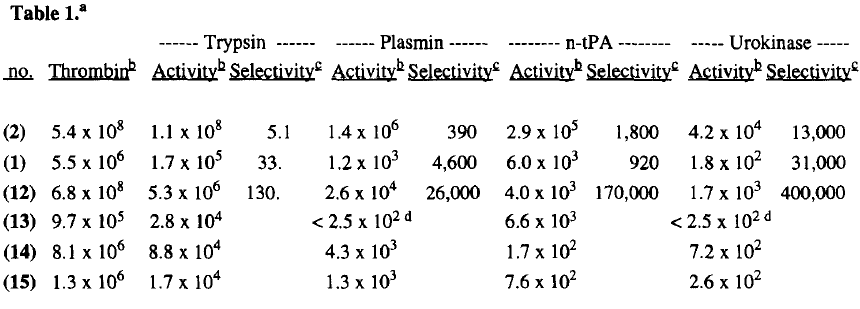
Its selectivity was gained by binding its nitrogens on the benzamidine by hydrogen bonding to Asp 189, and by fitting its benzamidine benzene into a hydrophobic pocket with Ser 214-Glu 217 and Asp 189-Glu 192 on the other side. The residue is in another hydrophobic pocket made up of His 57, Tyr 60A, Leu 99, and Trp 60D. The rest of this structure is unimportant on review of inhibitor 65 because only the amidinobenzylamine which occupies R1 and the proline which occupies the R2 sit are the same residues on inhibitor 65.[5]
P3 and P4 of Inhibitor 65
Inhibitor 65 additionally has a sulfonyl group with a methoxy group at the end of the residue. The residue sits in a hydrophobic . It sits in tight VanderWaals structure with the surrounding residues Leu 99, Tyr 60A, Arg 97, Glu 97, and Asn 98.The group holds its own VanderWaal forces. Additionally, the points to the carbonyl oxygen of Asn 98 and there is a weak Cl-π bond with Trp 215. For the P3 , there the backbone of asparagine forms and anti parallel beta sheet with Gly 216. The alkylated asparagine’s NH binds to a water molecule which then connects to Glu 192, and the hydrogens of Gly 219 and Gly 216.
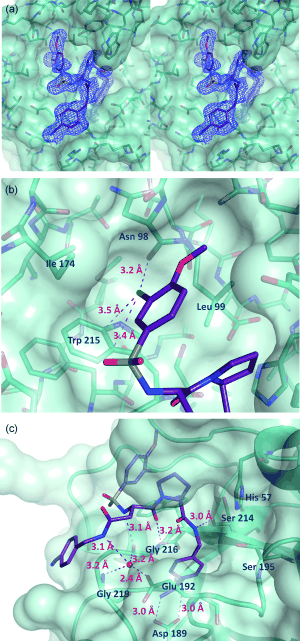
</StructureSection> This is the of thrombin
References
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ http://voices.yahoo.com/what-fibrinolytic-enzymes-6186058.html?cat=68
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428
- ↑ M. R. Wiley, N. Y. Chirgadze, D. K. Clawson, T. J. Craft, D. S. GiffordMoore, N. D. Jones, J. L. Olkowski, L. C. Weir, G. F. Smith, Bioorg. Med. Chem. Lett. 1996, 6, 2387. http://www.sciencedirect.com/science/article/pii/0960894X96004428
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ Steinmetzer T, Baum B, Biela A, Klebe G, Nowak G, Bucha E. Beyond Heparinization: Design of Highly Potent Thrombin Inhibitors Suitable for Surface Coupling. ChemMedChem. 2012 Aug 20. doi: 10.1002/cmdc.201200292. PMID:22907907 doi:10.1002/cmdc.201200292
- ↑ January 2002 Molecule of the Month by David Goodsell http://www.rcsb.org/pdb/101/motm.do?momID=25