Telomerase Reverse Transcriptase
From Proteopedia
Contents |
2b2a - The TEN domain of the Telomerase Reverse Transcriptase of Tetrahymena Thermophila
Introduction
Telomerase is a ribonucleoprotein enzyme. It adds specific telomeric DNA repeats to the 3'ends of linear chromosomes.
It was discovered in 1985 by Freider and Blackburn in Tetrahymena. Basically the Telomerase includes an RNA (TR) subunit and a subunit called Telomerase Reverse Transcriptase (TERT). The function of the TR subunit is to provide the template for telomeric DNA synthesis, which is done by the TERT.
The structure of TERT is similar to other reverse transcriptases in their catalytic domain. In contrast to other reverse transcriptases the TERTs have a large N-terminal extension which is called TERT essential N-terminal (TEN) domain. [1]
TEN form stable interactions with the telomerase RNA (TR) subunit.
Binding of the RNA subunit is possible, because of the presence of TEN in TERTs.
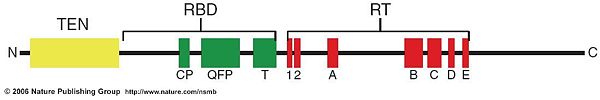
TEN: TERT essential N-terminal domain RBD: RNA-binding domain RT: Reverse-transcriptase domain
Experimental fragment with TEN
|
A Tetrahymena Thermophila TERT fragment consisting of residues 2-191 with an N-terminal His6 tag was cristallized in space group P3 with TERT molecules present in the crystallographic asymmetric unit. This 23.5-kDa fragment contains only one methionine residue, making phasing by substitution with selenomethionine (SeMet) difficult. Therefore, additional were engineered into the protein sequence for phasing purposes. The TEN domain is a monomer in solution (T.thermophila telomerase is monomeric) and the three TEN domain molecules are identical (for example average r.m.s. deviation of 0,0095A). The of each is the region, which varies among the three TEN molecules. Therefore this region (residues 177-191) is flexible in solution and needs a macromolecular partner for exact folding.
Structure
Overall Structure
This domain is essential for the enzymatic activity. It interacts specifically with and provides an anchor site for telomeric DNA substrates. It functions as a non-sequence-specific RNA-binding domain.
The TEN domain is a mixed . The core is a β-sheet,which consists of four antiparallel . This β-sheet is surrounded by seven and a short . β2 is connected with α4 (residues 77-87) and β4 with α5 (residues 122-127) through loops. The methionine can be found at either end of α4. Loops also link the His6 tag and the N-terminal TERT residues 2-12.
The TEN domain shows topologically two region of fold. The first is an N–terminal region, including α1, α2, α3, β1, and β2. The second is a C-terminal region, consisting of α4, α5, α6, α7, β3, β4, β5 and β6 (secondary structure elements). Although these two regions are distinct, they are anchored together. This is possible, because of numerous hydrophobic and interaction between elements β2 and β5, α3 and α6, and α2 and α5. That buries 2,706 A² of surface area.
The two regions are depended from each other for the correct folding.
Comparison with TERT homologes
There are three residues which are conserved in all known TERT stuctures. These residues are Gly144, Gln168 and Gly171. Both glycine residues adopt φ/ψ angles that are only possible for glycine and seem to be crucial for the folding of the TEN domain.
conects α5 and α6 allowing for an extremely tight turn to occur between these helices.
conects β6 and α7, allowing a sharp turn to avoid a steric clash with α6.
is exposed on the surface of the TEN domain and is important for catalytic activity.
Importance of TEN
Deletion of the entire TEN domain rendered telomerase completely inactive. Gln168, Phe178, Trp187 are required to properly bind or orient the DNA primer. The C-tail and the N-tail are implicated in RNA bindind. Therefore is TEN essential for telomerase activity. It is proposed that TEN is necessary for proper assembly or orientation of telomerase RNA-DNA complex in the TERT active site.
Discussion
Development of cancer chemotherapeutics directed against the reverse-transcriptase domain of telomerase has been challenging, perhaps because the enzyme needs only low activity to maintain telomeres. Because the TEN domain is essential for telomerase activity and its structure seems to be conserved trough evolution, this portion of TERT is a potential new target for antitelomerase compounds.
3D structures of telomerase reverse transcriptase
See Telomerase
Additional Resources
For additional information, See: Cancer
References
- ↑ Jacobs SA, Podell ER, Cech TR. Crystal structure of the essential N-terminal domain of telomerase reverse transcriptase. Nat Struct Mol Biol. 2006 Mar;13(3):218-25. Epub 2006 Feb 5. PMID:16462747 doi:10.1038/nsmb1054
User contributions
This page was created with content from a page authored by User:Tanja Emmerich and User:Vinzenz Alejandro Bayro Kaiser.
Proteopedia Page Contributors and Editors (what is this?)
Eran Hodis, Michal Harel, David Canner, Alexander Berchansky
