User:Alexander Rudecki/Sandbox 1
From Proteopedia
Contents |
Introduction
Drosophila melanogaster glutaminyl cyclase (DromeQC; also known as CG10487, CG32412 or Dmel\CG32412) is a globular protein part of the α/β-hydrolase superfamily[1]. DromeQC is an aminoacyltransferase (EC 2.3.2.5) that acts on N-terminal glutamine or glutamate residues, producing a protease-resistant cap in substrate proteins[1]. The human orthologue of DromeQC (hQC) has been implicated in stabilizing amyloid Aβ peptides involved in neurodegenerative disorders such as Alzheimers[2]. It has been shown that DromeQC has a similar overall fold to hQC, as well as a conserved active site[1]. Thus, DromeQC is an attractive candidate for transgenic models and mechanistic studies, which may further characterize neurodegenerative disorders and help develop treatments.
DNA--> RNA--> Protein
DromeQC is encoded by chromosome 3L, locus 64F4-64F5 in the D. melanogaster genome[3]. This gene is transcribed into a 1622 nucleotide transcript, containing 5' (36 nucleotides) and 3' (83 nucleotides) untranslated regions [3]. The translated protein contains 340 residues corresponding to a mass of 38,028 Da[4]. It also contains a 27 residue signal sequence, suggesting its involvement in the secretory pathway [2].
Protein Family
DromeQC belongs to the α/β-hydrolase fold superfamily; this superfamily exhibits a β-sheet core (5-8 strands) connected to α-helices, forming an α/β/α sandwhich[5]. The α/β-hydrolases are well characterized in the ESTHER database (ESTerases and α/β-Hydrolase Enzymes and Relatives), which as of present contains 168 protein families[6]. Most of the proteins in this superfamily function via a conserved catalytic triad - a nucleophile, acid and base - which is present on the loops of the active site[5]. Further information on catalysis is found in the 'Catalytic Mechanism' section on this page.
It is interesting that DromeQC prevents protein degradation from aminopeptidases, yet theses two enzymes share a common fold and active site[7]. This suggests that the enzymes act in a similar manner, with opposing effects on substrate.
Structure
|
Visual Walkthrough
The following descriptions can be viewed in the 3D JSmol image when any of the green links are clicked (Figure 1). DromeQC is a consisting of an A Chain and a B Chain. This homodimer can also be viewed in and representations. The backbone of DromeQC can be easily seen as an . Here, polypeptide progression in each chain is depicted by the following template:
| Amino Terminus | Carboxy Terminus |
The of DromeQC consists of either Protein, Ligand, or the Solvent in which it was crystallized (water). When DromeQC is colour coated (Alpha Helices, Beta Strands ) the global fold may be visualized nicely. This fold is driven by residues. The majority of Hydrophobic residues lie within the enzyme's interior, consistent with the hydrophobic collapse folding theory. In contrast, most Polar residues reside on the exterior where they contact the polar solvent. Similarly, , either Anionic (-) or Cationic (+), appear to cluster on the outside of the protein. From analysis of charged residues, it can be seen that a salt bridge may connect the two monomers (Chain A→Chain B: ARG35→GLU64).
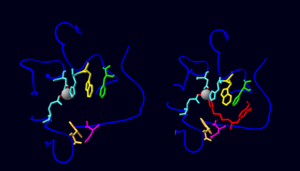
![Figure 2. A 3D graphical representation displaying the homodimer glutaminyl cyclase from Drosophila melanogaster (PDB: 4F9U). Secondary structure is depicted by red (α-helix) and yellow (β-strand) ribbons, glycosyl groups are coloured pink, while hydrogen bonds between the two monomers are shown by dotted green lines. The active site of DromeQC contains a chelated zinc ion represented by a gray sphere. Also bound to the active site and depicted as blue is the inhibitor 1-(3,4-dimethoxyphenyl)-3-[3-(1H-imidazol-1-yl)propyl]thiourea.](/wiki/images/thumb/7/72/Cropped_dimer.jpg/400px-Cropped_dimer.jpg)
Topology and Overall Structure[1]
As shown above, DromeQC is made up of 2 identical, independent monomers that come together to form an asymmetric (Figure 1). The subunits are connected via 4 hydrogen bonds (Chain A→Chain B: ARG35 NH2→GLU64 OE2, ARG43 NH2→ASN71 O, ARG43 NH2→PHE75 O, ARG43 NH1→PHE75 O) and surface complementarity (Figure 2). The subunits exhibit a globular α/β-hydrolase fold, characterized by a central twisted β-sheet motif consisting of 5 parallel strands (β1 and β3-β6) and an antiparallel β2 strand (Figures 2&3). This β-center is flanked by 9 surrounding α-helices; 2 fill the concave face (α5, α7), 7 fill the convex face (α1- α5, α8, α9) with one helix at the edge (α6) of each monomer. DromeQC is glycosylated (with up to 7 carbohydrate moieties) at the N42 position. These polysaccharide tags increase the solubility of DromeQC, and appear to have no affect of protein activity.
Within each subunit is 1 disulfide bond (C113→C136) linking the β3 strand to the α3 helix. These cysteine residues are situated close to the active site and are conserved in the human orthologue, suggesting a pivotal role in catalysis[2]. However, this hypothesis was ruled out when cysteines were replaced with alanines via site-directed mutagenesis; in these experiments no kinetic differences were observed in the DromeQC mutant[1]. In contrast, these mutations did affect structural differences of DromeQC, as determined by thermal unfolding experiments[1]. These results correspond to the structural stabilization of this disulfide bond in hQC, with no apparent effect on kinetic activity[9].
Active Site
The active site of DromeQC is located on four loops that lack secondary structure (Figure 4). Using these loops as a scaffold, a catalytic zinc ion is chelated via D131 OD2, E171 OE2, and H297 NE2. Thus, under the absence of substrate or inhibitor, Zn2+ exhibits trivalency (Figure 4). However, when DromeQC was crystalized in presence of a PBD150 inhibitor, Zn2+ was additionally chelated by the PBD150 imidazole moiety[1]. In a similar fashion, it is plausible that the γ oxygen of glutamine in peptide substrates chelates Zn2+. This would lead to proper position, polarization, and stabilization of substrate for cyclization.
Inhibitor binding[1]
Binding of PBD150 to DromeQC utilizes π-π, arene-H, and hydrogen bonding interactions (Figure 4). The dimethoxyphenyl phenyl group of PBD150 is stabilized by π-π interactions with F292. This phenyl group is highly flexible, however, as only weak electron density was observed during crystal structure analysis. Such flexibility could be essential for substrates to cyclize. Also stabilizing the inhibitor is an arene-H interaction made between the imidazole moiety of PBD150 and W296. The first carbon upstream of this imidazole ring forms an additional arene-H contact with W176. Finally, the sulfur contained in the thiourea group makes a hydrogen bond with D271. This sulfur could mimic a carbonyl oxygen in the backbone of a peptide, suggesting a possible substrate binding mechanism.
Function
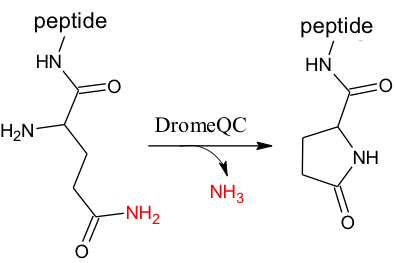
The N-terminus of many proteins (ie gonadotropin releasing hormone and thyrotropin-releasing hormone) contain a pyroglutamic acid (pGlu) residue[10] (Figure 5, right). A pGlu ‘cap’ protects these proteins against degradation by aminopeptidases, and influences the conformation of the hormone or its associated receptor, leading to their activation[2]. Cyclization also leads to decreased basicity in the peptide. Though cyclization of Gln-tRNA to pGlu-tRNA has been shown to occur in papaya latex,[11] N terminal pGlu formation must be post translational due to an essential methionine that initiates translation in all organisms.
Catalytic Mechanism[12]
DromeQC is responsible for the post-translational processing of polypeptides. DromeQC catalyzes the cyclization of N-terminal glutamine, and to a lesser extent glutamate, into pyroglutamic acid (pGlu, 5-oxo-L-proline, or <Glu) (Figure 5). This enzyme can be categorized as follows:
- -transferase (2)
- -acyltransferase (2.3)
- -aminoacyltransferase (2.3.2)
- -acts on glutaminyl/glutamyl residues (2.3.2.5)
- -aminoacyltransferase (2.3.2)
- -acyltransferase (2.3)
The cyclization reaction occurs via a nucleophilic attack of the N-terminal α-amine on the γ carbon in the glutamine side chain. The enzymatic mechanism for DromeQC is still undetermined, but it seems plausible that it follows that of its human orthologue. In hQC, the N-terminus of the peptide substrate is inserted into the active site pocket, where the γ amide oxygen chelates the catalytic zinc ion. This ion-dipole interaction causes carbonyl polarization, making it a better electrophile. To facilitate the reaction, a conserved glutamate (Glu201) acts as both a general base and acid. Glu201 abstracts a proton from the α-amine group, causing it to nucleophilically attack the γ amide oxygen. This produces a tetrahedral intermediate with a charged oxygen that is stabilized by Zn2+. Glu201 then protonates the γ amide nitrogen, and an amine group is expelled as the carbonyl reforms. Also essential to this mechanism is a conserved aspartate (Asp248) that coordinates/stabilizes the leaving amine group.
Localization
DromeQC is known to localize in the brain and peripheral nerves of D. melanogaster[2]. This fact was unveiled by the discovery of adipokinetic hormone; this protein has an N-terminal pGlu, supporting a post translational modification via DromeQC[13]. Adipokinetic hormone was found to localize in neurons and nerve endings by immunohistochemistry, functioning as neuromodulator in tissues such as heart and skeletal muscle[14]. Thus, It has been suggested that DromeQC is part of the secretory pathway, being targeted to secretory vesicles where hormone maturation takes place[2]. This claim is supported by a 27 residue signal sequence contained at the N-terminus of DromeQC[2]. Further support stems from immunohistochemical evidence that DromeQC and modified substrate are excreted into the extracellular medium[2].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Koch B, Kolenko P, Buchholz M, Ruiz Carrillo D, Parthier C, Wermann M, Rahfeld JU, Reuter G, Schilling S, Stubbs MT, Demuth HU. Crystal Structures of Glutaminyl Cyclases from Drosophila melanogaster Reveal Active Site Conservation between Insect and Mammalian QCs. Biochemistry. 2012 Aug 16. PMID:22897232 doi:10.1021/bi300687g
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat Med. 2008 Oct;14(10):1106-11. doi: 10.1038/nm.1872. Epub 2008 Sep 28. PMID:18836460 doi:http://dx.doi.org/10.1038/nm.1872
- ↑ 3.0 3.1 DromeQC Gene Card. NCBI. [1]
- ↑ DromeQC. UniProt. [2]
- ↑ 5.0 5.1 Lenfant N, Hotelier T, Velluet E, Bourne Y, Marchot P, Chatonnet A. ESTHER, the database of the alpha/beta-hydrolase fold superfamily of proteins: tools to explore diversity of functions. Nucleic Acids Res. 2013 Jan;41(Database issue):D423-9. doi: 10.1093/nar/gks1154. , Epub 2012 Nov 27. PMID:23193256 doi:http://dx.doi.org/10.1093/nar/gks1154
- ↑ Lenfant, N., Hotelier, T., Velluet, E., Bourne, Y., Marchot, P., and A Chatonnet. (2013) ESTHER, the database of the alpha/beta-hydrolase fold superfamily of proteins: tools to explore diversity of functions. Nucleic Acids Research 41: D423-9. [3]
- ↑ Booth RE, Lovell SC, Misquitta SA, Bateman RC Jr. Human glutaminyl cyclase and bacterial zinc aminopeptidase share a common fold and active site. BMC Biol. 2004 Feb 10;2:2. PMID:15028118 doi:10.1186/1741-7007-2-2
- ↑ PDB Sum Entry 4F9U. EMBL-EBI. [4]
- ↑ Ruiz-Carrillo, D., Koch, B., Parthier, C., Wermann, M., Dambe, T., Buchholz, M., Ludwig, H., Heiser, U., Rahfeld, J., Stubbs, M. T., Schilling, S., and H. Demuth. (2011) Structures of glycosylated mammalian glutaminyl cyclases reveal conformational variability near the active center. Biochemistry. 50: 6280-6288. DOI: 10.1021/bi200249h
- ↑ Goren HJ, Bauce LG, Vale W. Forces and structural limitations of binding of thyrotrophin-releasing factor to the thyrotrophin-releasing receptor: the pyroglutamic acid moiety. Mol Pharmacol. 1977 Jul;13(4):606-14. PMID:196172
- ↑ Bernfield MR, Nestor L. The enzymatic conversion of glutaminyl-tRNA to pyrrolidone carboxylate-tRNA. Biochem Biophys Res Commun. 1968 Dec 9;33(5):843-9. PMID:4881333
- ↑ Calvaresi M, Garavelli M, Bottoni A. Computational evidence for the catalytic mechanism of glutaminyl cyclase. A DFT investigation. Proteins. 2008 Nov 15;73(3):527-38. doi: 10.1002/prot.22061. PMID:18470930 doi:http://dx.doi.org/10.1002/prot.22061
- ↑ Schaffer MH, Noyes BE, Slaughter CA, Thorne GC, Gaskell SJ. The fruitfly Drosophila melanogaster contains a novel charged adipokinetic-hormone-family peptide. Biochem J. 1990 Jul 15;269(2):315-20. PMID:2117437
- ↑ Schooneveld H, Tesser GI, Veenstra JA, Romberg-Privee HM. Adipokinetic hormone and AKH-like peptide demonstrated in the corpora cardiaca and nervous system of Locusta migratoria by immunocytochemistry. Cell Tissue Res. 1983;230(1):67-76. PMID:6342796