User:David McDonald/Replication Termination Protein
From Proteopedia
As with most bacteria, DNA replication of the circular chromosome of B. Subtilis occurs in a bi-directional fashion, starting from a common origin of replication (ori) and ending in the termination region, approximately 180o from the ori. The two replication forks are forced to meet in the termination region by replication termination proteins (RTPs) complexed to specific, unidirectional DNA termination sits (Ter sequences) termination region and arrest the action of the replication fork in a directional manner. That is, there are RTP:Ter complexes which stop replication in the clockwise and in the anti-clockwise direction. The clockwise replication fork is unaffected by the RTP:Ter complexes for the anti-clockwise fork and vice versa.
Contents |
DNA Replication and Termination in B. Subtilis
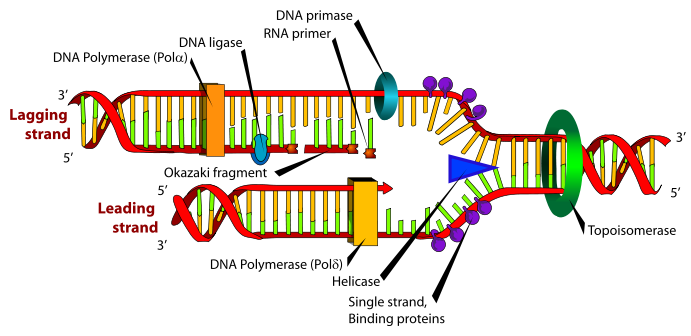
Diagram of the bacterial replisome
B. Subtilis is used as a model organism in bacterial DNA replication. Once replication is initiated, two replisomes are recruited, one travelling clockwise and one anti-clockwise around the circular chromosome. The two strands of DNA (the leading and lagging strands) which emerge following the passage of the replisome give rise to the name “replication fork” often used when referring to an active replisome.
The replisome contains a large array of proteins working collectively to copy the DNA. Three major functions are required[1]: 1. Unwinding and separation of the existing DNA strands” Topoisomerase is responsible for unwinding the incoming DNA, whilst helicase breaks the hydrogen bonds between base-pairs, allowing the DNA to become single-stranded. The action of helicase is believed to be the step interrupted by RTP during replication termination.[2]
2. Synthesis of DNA by polymerases: DNA pol III “reads” one of the two strands of ssDNA released by helicase and creates a complimentary strand, giving rise to dsDNA.
3. A method of copying the “lagging strand”, which is opposite to the directionality of the polymerases: DNA pol III can only create DNA in a 5’-3’ fashion. Since one of the two DNA strands to be replicated (the lagging strand) is 3’-5’, a mechanism is needed of overcoming this problem. DNA primers create short ds-DNA ~2kbp from the 5’ end of the replicated DNA which DNA pol III can continue. Since this leads to long stretches of ssDNA, ssDNA binding proteins are recruited to protect the ssDNA. These fragments are known as Okazaki fragments, and are joined by DNA ligases.
Structure
|
RTP contains 122 amino acid residues and is an example of a winged helix structure, in the α+β protein folding family, containing four α-helices and two β-strands[3]. The structure is named a "winged helix" due to the central helical region, flanked by "wings" comprised of the two β-strands and the loops between them.
In a cell, RTP exists as a homodimer, where the monomer subunits are tightly associated through antiparallel coiled-coil interactions between the C-terminal α4 helices to form the functional Dimer. This dimer is held together by hydrophobic interactions between the two α4 helices.
For crystallographic studies, a mutant of RTP (C110S) was created to avoid possible unwanted disulfide bond formation. In this mutant, the Cysteine at position 110 was changed to a Serine residue. It was shown that this did not result in a change in solution dimerisation or DNA binding functionality[4].
DNA Binding
|
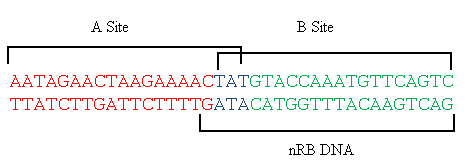
In order to terminate chromosomal replication, RTP must complex with the DNA. Two dimers of RTP bind at the Ter site, a 37bp region of DNA containing two pseudosymmetric overlapping 21 base pair half-sites A and B[5]. The B site displays significantly higher affinity for the RTP dimer[6], however it has been shown that it is necessary for both A and B to be occupied in order for replication fork termination to occur.[6]
The formation mechanism and subsequent structure this RTP-DNA complex has been a topic of some debate. Previously, it was thought that the complex was symmetric[7], with DNA interaction mediated by the α3 helix as predicted, but also the α1 helix and, surprisingly, the α1-α2 loop region. More specifically, the binding was attributed to residues Arg59, His54, Thr55 and Tyr58 of α3; Tyr33, Gly34 and Leu35 of α 1- α2 loop region and Gln15 of α1[7]. This structure was obtained with a symmetric B site analogue known as sRB as opposed to the wild type Ter sequence, which differs by 6 nucleotides. In particular, three modified upstream residues in sRB lie govern the overall interaction with the α3 helix. It is theorised that this changed interaction resulted in the initial, but incorrect, symmetric binding mode. [5]
The observed symmetry was in contrast to the known polarity of the RTP-Ter site. Further research using the wild-type Ter sequence (nRB) revealed a distinct asymmetry in both the RTP dimer and the DNA[5]. Although the interacting sequences remained the same, the downstream monomer displayed a "Wing down" confirmation, where the β2-loop-β3 structure makes contact with the phosphate backbone. Conversely, the upstream monomer displays a "Wing up" confirmation, with the wing making contact with the upstream minor groove. It is also proposed that interactions between these wings result in the co-operative binding properties of the Ter site.[4]
DNA map of the TerI site in B. Subtilis 168. RTP binding sites A and B are indicated, as well as nRB, the DNA sequence used for crystallographic studies of the asymmetric complex. DNA sequence adapted from Vivian et al (2007)[4].
Replication Termination
As mentioned above, Ter sites of DNA contain two overlapping binding domains for RTP dimers. The B site (which has a higher affinity for RTP) is filled first, and co-operative protein interactions between the RTP:B site complex recruit a second RTP dimer into the A site. Smith and Wake[8] showed that the complex exhibits a polar termination, whereby the replisome was only halted when approaching RTP:Ter from the B site, and that occupation of neither the A or B sites alone were sufficient to arrest replication. There are competing theories as to the mode of action through which RTP interrupts the function of the replisome with the correct directionality, yet it is widely believed that RTP arrests the function of DNA helicase2.
Since changes to the DNA-binding affinity of RTP limit its ability to terminate replication[9], A mechanism for RTP action was proposed where the strength of the DNA binding of RTP created a molecular "clamp", such that the helicase machinery could not separate the DNA strands. This failed, however, to account for the directionality of RTP action. Further evidence against the clamp model was provided by Duggin in 2006[2], showing that mutant RTP containing extra polypeptide chains but the same DNA binding affinity had a reduced termination activity. Instead, the model suggested was that with specific protein:protein interactions between RTP and helicase result in termination. Since the binding of RTP in the B site was long believed to be symmetric[7], the model failed to account for the observed termination polarity.
The asymmetry found in the native B. Subtilis Ter site[5] provides a possible mechanism for the polarity of RTP. The "Wing up" and "Wing down" sites, respectively, bind to the minor groove and the phosphate backbone of DNA. A plausible model is that RTP is only in the correct conformation to interact with helicase in one of the two sides. Further research is required to confirm this model.
Works Cited
1. K. Marians (2008) “Understanding how the replisome works” Nat. Struct. Mol. Biol. 15:125-127
2. I.G. Duggin, (2006). "DNA Replication Fork Arrest by the Bacillus subtilis RTP–DNA Complex Involves a Mechanism that Is Independent of the Affinity of RTP–DNA Binding". J Mol Biol. 361(1):1-6.
3. Bussiere, DE, Bastia, D and White, SW. (1995)Crystal structure of replication terminator protein of B. subtilis at 2.6 Å. Cell. 80: 651-660.
4. J.P. Vivian, A.F. Hastings, I.G. Duggin, R.G. Wake, M.C.J. Wilce, and J.A. Wilce (2003) “The impact of single cysteine residue mutations on the replication terminator protein” Biochem. Biophys. Res. Commun. 310(4):1096-1103
5. J. P. Vivian, C. J. Porter, J. A. Wilce, M.C. J. Wilce (2007) “An Asymmetric Structure of the Bacillus subtilis Replication Terminator Protein in Complex with DNA” J. Mol. Biol. 370:481-491
6. I. Duggin, J. Matthews, N. Dixon, R. Wake, J. Mackay (2005) “A Complex Mechanism Determines Polarity of DNA Replication Fork Arrest by the Replication Terminator Complex of Bacillus subtilis” J. Biol. Chem. 280(13):13105-13113
7. J.A. Wilce, J.P.Vivian, A.F. Hastings, G. Otting, R.H.A. Folmer, I.G. Duggin, R.G. Wake, M.C.J. Wilce (2001) “Structure of the RTP–DNA complex and the mechanism of polar replication fork arrest” Nat. Struct. Biol. 8(3):206-210
8. M.T. Smith and R.G. Wake (1992)"Definition and polarity of action of DNA replication terminators in Bacillus subtilis". J. Mol. Biol. 227, 648–657
9. I. G.Duggin, P. A. Andersen, M. T. Smith, J. A. Wilce, G. F. King, R. G. Wake (1999). "Site-directed mutants of RTP of Bacillus subtilis and the mechanism of replication fork arrest." J Mol Biol. 286(5):1325-35.