User:Ona Ambrozaite/Sandbox 1
From Proteopedia
Contents |
Trichosurin
|
Introduction
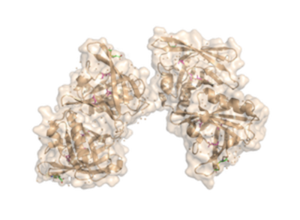
Trichosurin is one of three predominant proteins found in the milk whey of the common brushtail possum Trichosurus vulpecula. It belongs to the lipocalin superfamily and is an extracellular protein (17–25 kDa) that can bind and transport small lipophilic molecules. The binding pocket is a crucial factor in the selectivity of binding, as is access to the binding pocket that is controlled by side chains of the 22 residues that line the binding pocket and form a loop between β-strands at one end of the lipocalin β-barrel. Trichosurin is most closely related to the major urinary proteins (MUPs) from mice and rats and is a significant constituent of milk throughout possum lactation [1].
Structure
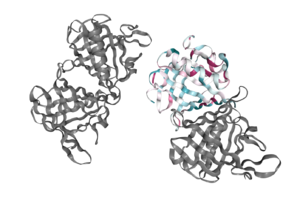
The fully functional trichosurin consists of two monomers joining to form a of identical eight-stranded, anti-parallel β-barrels [2]. The study of lipocalin dimerization modes has shown six variations in orientation, with trichosurin adding a seventh one to the list. Overall, there are four main chains (A, B, C, and D) in the dimer, with each chain a length of 166 amino acids. There is also a signal sequence at the N-terminus of the chain that is cleaved before a fully functional molecule is formed. Currently, there are two crystal structures of trichosurin that can be found at RCSB, one determined at pH 8.2 and another at a lower pH of 4.6. With regards to the binding cavity, there are the , which lie in the pocket and offer hydrogen-bonding sites for a potential ligand, especially Asn49. The shape and charge distribution within the pocket suggest that small hydrophobic molecules can be accommodated within, with such sites providing the necessary hydrogen bonding opportunities.
The SCOP website classifies trichosurin as a fatty acid retinoid binding protein, just like the nematode fatty acid retinoid binding protein from Necator americanus species. Trichosurin belongs to the calycin beta-barrel core domain superfamily, as indicated by the search results on the CATH website, which emphasizes the presence of the β-barrels throughout the structure.
Structure and Function
|
As expected for an extracellular protein, trichosurin displays a large amount of hydrophilic , with the more hydrophobic areas within the barrels themselves and between the helix, the outside of the barrel, and the dimer interface where ligands such as 2-naphthol can bind. The binding site is found in the centre of the β-barrel and can be occupied by water and isopropanol molecules from the corresponding crystallization medium. In fact, researchers were able to crystallize trichosurin with 2-naphthol and 4-ethylphenol, the presence of which provides clues to the function of trichosurin.
Overall, trichosurin follows the general lipocalin superfamily fold pattern by having a β-barrel, several extended loop regions between β strands that close the ends of the barrel, and a helix near the C-terminus which is common to all lipocalins. There's also a major conserved feature of the lipocalin fold, a disulfide bond, formed by in each chain that ties the C-terminus to the corresponding β-strand. There are also N-linked glycosylations on Asp67 and Asp148 that could help keep trichosurin in solution, which is important since it is a secreted protein that needs to remain soluble and not aggregate. It could also facilitate the folding process and exert quality control to monitor trichosurin's folding.
The binding preference for small phenolic compounds such as 2-naphthol and 4-ethylphenol with high Kd values signifies that very similar compounds can have higher affinities and act as natural ligands of trichosurin as well.
Evolutionarily Related Proteins
Trichosurin has 34% amino acid identity to the rodent major urinary proteins (MUPs ) and 28% amino acid identity to the major horse dander allergen equc1. Tichosurin homologues have been identified in both the tammar wallaby and the South American opossum (77% amino acid identity). Such high level of conservation in species that diverged about 80 million years ago suggests that the function of trichosurin is critical in metatherian lactation.
With regards to structural comparison with other lipocalins such as bovine BLG that is a prototypic milk lipocalin, trichosurin shows great similarity. This is because structural alignment of trichosurin, expressed in E. coli and crystallized at pH 4.8, with the BLG structure 1BEB yielded an rmsd of 2.76 Å over 139 equivalent residues.
Relevance
Trichosurin, an important marsupial milk protein, is highly conserved across metatherians. The opossum, a metatherian, shared a common ancestor with humans around 130 million years ago and is of particular importance to study the differences between the development of metatherians and other mammals. In opossums, lactation is divided into three stages, each has a very unique milk composition, and trichosurin is one of three predominant lipocalins found in the milk of T. vulpecula [3], [4].
It is known that phenolic compounds are found in plants and used to deter herbivores, including possums, who then have to metabolize these compounds. It has also been shown that female possums concentrate phenolic compounds in their milk, which could act as a priming source for the young's liver to produce detoxifying enzymes that they can then use to survive while eating toxic plant sources.
When other plant phenolics such as cinneol and gallic acid were tested in binding trichosurin, they displayed size and hydrogen-bonding groups, which makes them appropriate ligands for trichosurin. Researchers also note that the hypothesis of phenolic compounds priming detoxification pathways would explain the high level of sequence conservation of trichosurin among metatherians.
Available 3D Structures
References
- ↑ Watson RP, Demmer J, Baker EN, Arcus VL. Three-dimensional structure and ligand binding properties of trichosurin, a metatherian lipocalin from the milk whey of the common brushtail possum Trichosurus vulpecula. Biochem J. 2007 Nov 15;408(1):29-38. PMID:17685895 doi:10.1042/BJ20070567
- ↑ Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000 Oct 18;1482(1-2):9-24. PMID:11058743
- ↑ Cowan PE. Changes in milk composition during lactation in the common brushtail possum, Trichosurus vulpecula (Marsupialia: Phalangeridae). Reprod Fertil Dev. 1989;1(4):325-35. doi: 10.1071/rd9890325. PMID:2636424 doi:http://dx.doi.org/10.1071/rd9890325
- ↑ Piotte CP, Hunter AK, Marshall CJ, Grigor MR. Phylogenetic analysis of three lipocalin-like proteins present in the milk of Trichosurus vulpecula (Phalangeridae, Marsupialia). J Mol Evol. 1998 Mar;46(3):361-9. doi: 10.1007/pl00006313. PMID:9493361 doi:http://dx.doi.org/10.1007/pl00006313
Additional Resources
See Fatty acid-binding protein for more information