From Proteopedia
proteopedia linkproteopedia link Malate Dehydrogenase
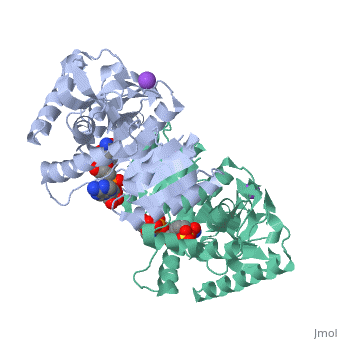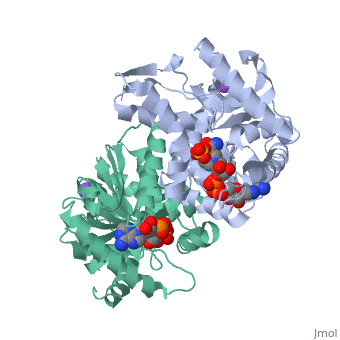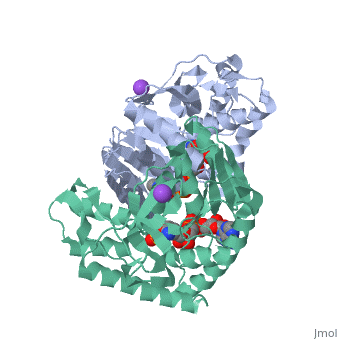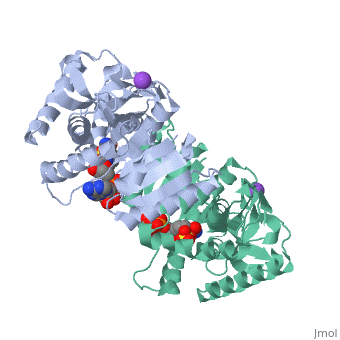
Malate Dehydrogenase (MDH)(PDB entry 2x0i) is most known for its role in the metabolic pathway in the tricarboxylic acid cycle[1]; however, the enzyme is also in many other metabolic pathways such as glyoxylate bypass, amino acid synthesis, glucogenesis, and oxidation/reduction balance [1]. It is classified as a oxidoreductase[2]. Malate Dehydrogenase has been extensively studied due to its many isozymes [2]. The enzyme exists in two places inside a cell, in the mitochondria and cytoplasm. In the mitochondria, the enzyme catalyzes the reaction of malate to oxaloacetate; but in the cytoplasm, the enzyme catalyzes oxaloacetate to malate to allow transport [3]. This conversion is an essential catalytic step in each different metabolic mechanism. The enzyme malate dehydrogenase is composed of either a dimer or tetramer [4].
The evolutionary past of MDH shows a divergence to form lactate dehydrogenase (LDH) which functions in a very similar way to MDH. Although there is a very low sequence conservation among MDH and LDH’s [3] the structure of the enzyme has remained relatively conserved. The key difference between the two is in the substrate: LDH catalyzes pyruvate to lactate.
|
|
| 2x0i, resolution 2.91Å ()
|
| Ligands:
| , ,
|
| Activity:
| Malate dehydrogenase, with EC number 1.1.1.37
|
| Related:
| 2x06, 2x0j, 2x0n, 2x0r, 2x0s
|
|
|
|
|
| Resources:
| FirstGlance, OCA, RCSB, PDBsum
|
| Coordinates:
| save as pdb, mmCIF, xml
|