11gs
From Proteopedia
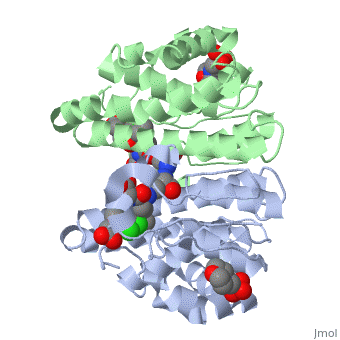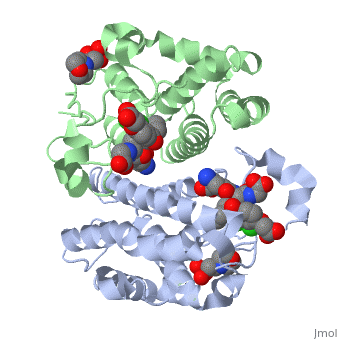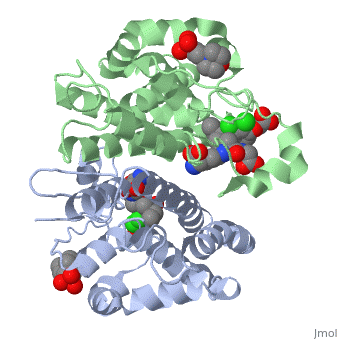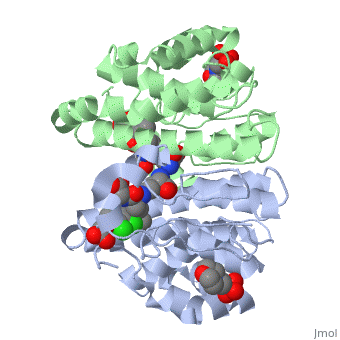
Glutathione s-transferase complexed with ethacrynic acid-glutathione conjugate (form ii)
Structural highlights
FunctionGSTP1_HUMAN Conjugation of reduced glutathione to a wide number of exogenous and endogenous hydrophobic electrophiles. Regulates negatively CDK5 activity via p25/p35 translocation to prevent neurodegeneration.[1] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe diuretic drug ethacrynic acid, an inhibitor of pi class glutathione S-transferase, has been tested in clinical trials as an adjuvant in chemotherapy. We recently solved the crystal structure of this enzyme in complex with ethacrynic acid and its glutathione conjugate. Here we present a new structure of the ethacrynic-glutathione conjugate complex. In this structure the ethacrynic moiety of the complex is shown to bind in a completely different orientation to that previously observed. Thus there are at least two binding modes possible, an observation of great importance to the design of second generation inhibitors of the enzyme. The glutathione conjugate of ethacrynic acid can bind to human pi class glutathione transferase P1-1 in two different modes.,Oakley AJ, Lo Bello M, Mazzetti AP, Federici G, Parker MW FEBS Lett. 1997 Dec 8;419(1):32-6. PMID:9426214[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||||