2p1q
From Proteopedia
Mechanism of Auxin Perception by the TIR1 ubiquitin ligase
Structural highlights
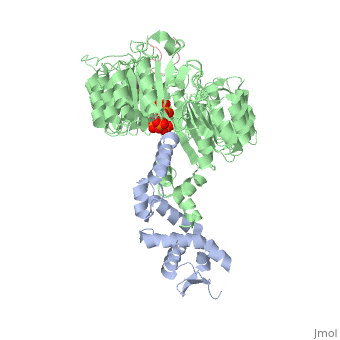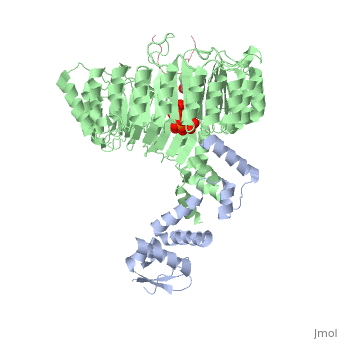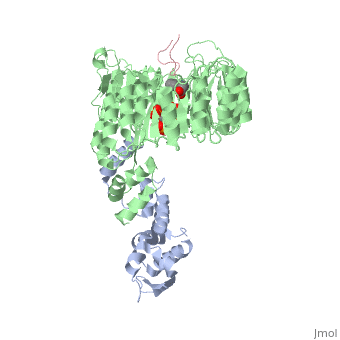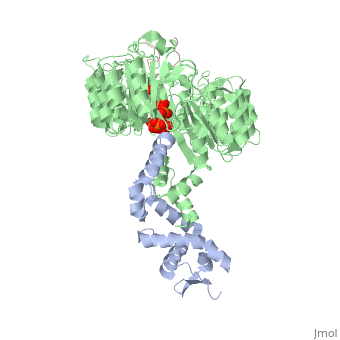
FunctionIAA7_ARATH Aux/IAA proteins are short-lived transcriptional factors that function as repressors of early auxin response genes at low auxin concentrations. Repression is thought to result from the interaction with auxin response factors (ARFs), proteins that bind to the auxin-responsive promoter element (AuxRE). Formation of heterodimers with ARF proteins may alter their ability to modulate early auxin response genes expression.[1] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedAuxin is a pivotal plant hormone that controls many aspects of plant growth and development. Perceived by a small family of F-box proteins including transport inhibitor response 1 (TIR1), auxin regulates gene expression by promoting SCF ubiquitin-ligase-catalysed degradation of the Aux/IAA transcription repressors, but how the TIR1 F-box protein senses and becomes activated by auxin remains unclear. Here we present the crystal structures of the Arabidopsis TIR1-ASK1 complex, free and in complexes with three different auxin compounds and an Aux/IAA substrate peptide. These structures show that the leucine-rich repeat domain of TIR1 contains an unexpected inositol hexakisphosphate co-factor and recognizes auxin and the Aux/IAA polypeptide substrate through a single surface pocket. Anchored to the base of the TIR1 pocket, auxin binds to a partially promiscuous site, which can also accommodate various auxin analogues. Docked on top of auxin, the Aux/IAA substrate peptide occupies the rest of the TIR1 pocket and completely encloses the hormone-binding site. By filling in a hydrophobic cavity at the protein interface, auxin enhances the TIR1-substrate interactions by acting as a 'molecular glue'. Our results establish the first structural model of a plant hormone receptor. Mechanism of auxin perception by the TIR1 ubiquitin ligase.,Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N Nature. 2007 Apr 5;446(7136):640-5. PMID:17410169[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. Loading citation details.. Citations No citations found See AlsoReferences
|
| |||||||||||||||||||