Allen sandbox 1
From Proteopedia
| |||||||||
| 1one, resolution 1.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Phosphopyruvate hydratase, with EC number 4.2.1.11 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
is an enzyme that catalyzes the reversible dehydration reaction of 2-phosphoglycerate (2PG) into phosphophenolglycerate (PEP) in the 9th reaction of glycolysis.[1] Glycolysis converts glucose into two 3-carbon molecules called pyruvate. The energy released during glycolysis is used to make ATP.[2] Enolase is expressed abundantly in most cells and has been proven useful as a model to study mechanisms of enzyme action and structural analysis, especially for those enzymes involved in glycolysis [3].
Contents |
Structure
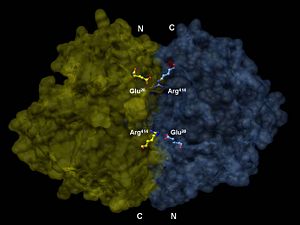
The of enolase contains both alpha helices and beta sheets. The beta sheets are mainly parallel[4]. As shown in the figure, enolase has about 36 alpha helices and 22 beta sheets (18 alpha helices and 11 beta sheets per domain), resulting in a molecular weight of 82,000-100,000 Daltons. Enolase consists of two domains paired together in antiparallel orientation.
Structural Clasification of Proteins (SCOP)[5]
Enolase is in the Alpha and Beta protein class (mainly parallel beta sheets) and has a fold of TIM beta/alpha-barrel, which consists of parallel barrels composed of beta sheets. Enolase also belongs to the Enolase C-terminal domain-like protein Superfamily, which means it can bind metal ions (magnesium or manganese) in a conserved site inside barrel N-terminal alpha+beta domains.
Mechanism
[6]The of enolase includes the residues Lys 345, Lys 396, Glu 168, Glu 211, and His 159. Enolase forms a complex with two at its active site. The mechanism of enolase follows 3 steps:
Step 1: The substrate, 2PG, binds to the two . The carboxyl group coordinates with the two magnesium ions, which stabilizes the negative charge on the oxygen atom and removes charge from the alpha hydrogen, making it a better leaving group.
Step 2: Lys 345 then deprotonates the alpha hydrogen, a reaction which is stabilized by resonance between the carboxyl oxygens and the two Mg ions and Glu 211 bonded to the hydroxyl group. This creates a carbanion intermediate.
Step 3: An electron transfer reaction then occurs from the C'1 carboxyl oxygen to form a ketone. This removes electrons from the alkene bond between C'1 and C'2 to create an alkene between C'2 and C'3 instead. This allows the C'3 hydroxyl group to deprotonate Glu 211, resulting in the ejection of a water molecule and formation of the product PEP. PEP is then dephosphorylated in the next step of glycolysis to create pyruvate.
Studies have shown that mutating the residues within the active site can have serious effects on the activity of the enzyme. For example, mutation in either Glu 168, Glu 211, Lys 345, or Lys 396 of the active site results in a reduction in the reaction activity by 10^4 to 10^5 of wild type levels. Clearly, these residues play critical roles in maintaining enolase's activity.[7]
Additionally, this mechanism can be inhibited by addition of Fluoride ions, which bond to Mg 2+, thus blocking the substrate (2PG) from binding to the active site of enolase.[8]
Kinetics
[9]The kinetics of the enolase reaction can be affected by the concentration of magnesium ion, Mg2+. The graph to the left shows velocity vs. [PGA], in which PGA stands for 2-PG. The upper curve is the reaction at normal [Mg2+], 0.01 M, while the lower curve shows the same reaction at an increased concentration of [Mg2+], 0.1 M. This shows that upon addition of Mg2+, the Vmax is lowered to sub-optimal levels, while the Km remains relatively unchanged. Therefore, the upper curve (lower [Mg2+]) is more desirable because it achieves a greater Vmax at essentially the same Km value; a greater Vmax can be attained without the need for additional substrate[10].
Regulation
Enolase is found on the surface of a variety of eukaryotic cells as a strong plamingoen-binding receptor and on the surface of hematopietic cells such as monocytes, T cells and B cells, neuronal cells and endothelial cells. Enolase in muscle cells can bind other glycolytic enzymes, such as phosphoglycerate mutase, muscle creatine kinase, pyruvate kinase, and muscle troponin, with high affinity. This suggests that enolase helps facilitate muscle contraction by creating a functional glycolytic segment in the muscle where ATP production occurs. Myc-binding protein (MBP-1) is similar to the α-enolse structure and is found in the nucleus as a DNA-binding protein[11].
Additional levels of enolase regulation occur through the addition of flouride ion, F-. F- forms a complex with Mg2+ already bound at the enzyme's active site. This blocks enolase from binding the substrate 2PG, causing it to build up and thereby slowing the process of glycolysis.
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008.
- ↑ Pancholi, V. "Multifunctional a-Enolase: Its Role in Diseases." CMLS, Cellular and Molecular Life Sciences 58 (2001): 902-20.
- ↑ The scop authors. Structural Classification of Proteins. “Protein: Enolase from Baker's yeast (Saccharomyces cerevisiae). 2009. 2/26 2010. [<http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.d.b.bc.b.b.html>.]
- ↑ The scop authors. Structural Classification of Proteins. “Protein: Enolase from Baker's yeast (Saccharomyces cerevisiae). 2009. 2/26 2010. [<http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.d.b.bc.b.b.html>.]
- ↑ Nguyen, Tram, and Katelyn Thompson. "Mechanism of Enolase Converting 2-Phosphoglycerate to Phosphoenolpyruvate." ChemDraw 10.0: Public Domain, 2008. [1].
- ↑ Reed, G., Poyner, R., Larsen, T., Wedekind, J., Rayment, I. (1996)Current Opinion in Structural Biology 6:736-743
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008.
- ↑ Westhead, E. W., and BO G. Malmstrom. "The Chemical Kinetics of the Enolase Reaction with Special References to the Use of Mixed Solvents." The Journal of Biological Chemistry 228 (1957): 655-71.
- ↑ Westhead, E. W., and BO G. Malmstrom. "The Chemical Kinetics of the Enolase Reaction with Special References to the Use of Mixed Solvents." The Journal of Biological Chemistry 228 (1957): 655-71.
- ↑ Pancholi, V. "Multifunctional a-Enolase: Its Role in Diseases." CMLS, Cellular and Molecular Life Sciences 58 (2001): 902-20.


![V vs. [PGA]; PGA is 2PG, the top curve has [Mg2+] of 10^-3 M and the bottom curve has [Mg2+] of 106-2 M](/wiki/images/thumb/1/10/Enolase_kinetics.jpeg/150px-Enolase_kinetics.jpeg)
