Alternate locations of backbones
From Proteopedia
At sufficiently high resolution, empirical methods for determining macromolecular structure may detect multiple locations for some atoms, termed alternate locations. When alternate locations for backbones (main chains) deviate substantially, the backbone may separate into two pathways ("fork") and then rejoin into a single pathway.
Contents |
Alternate location server
An Alternate Location Server (AltLoc Server) (part of the OCA Browser system) scans all entries in the Protein Data Bank, listing those that have alternate locations. The analysis on this page is based on an AltLoc Server report from late May, 2023, when the total number of entries in the PDB was ~200,700. Here are two example lines from the report:
ID Method Res Dep Date 9ins X-ray diffraction 1.70 1991-10-23 min=-1.0 ave=-1.0 max=-1.0 A=48 B=48 4dgd X-ray diffraction 1.4 2012-01-25 min=0.03 ave=4.78 max=15.87 A=84 B=84 A.CA=13 B.CA=13
- Res is the resolution in Å.
- Dep Date is the date of deposition into the PDB.
- min, ave, max are for the distances between AltLocs of backbone atoms only. "Backbone atoms" are protein alpha carbon atoms, and nucleic acid phosphorus atoms, as defined in the AltLoc Server. A value of -1 is given when no backbone atoms have AltLocs.
- A and B are the AltLoc code characters used in these cases. Any alphanumeric characters are legal, and atoms may have more than two AltLocs.
- The numbers after A= and B= are the numbers of atoms with A/B AltLoc codes.
- The numbers after A.CA= and B.CA= are the numbers of alpha carbon atoms with A/B AltLoc codes. For 9ins, the absence of .CA= counts means that no alpha carbons have AltLocs in this entry.
Prevalence of AltLocs
The prevalence of AltLocs is summarized in the article Alternate locations.
Backbone Altloc Separations
X-ray diffraction
Half of all X-ray diffraction entries have AltLocs, and ~90% of those (~79,000) have backbone atom AltLocs. However, only 1,035 entries with backbone atom AltLocs (1.3%) have "substantial" maximum backbone atom AltLoc separations of >5.0 Å. (The van der Waals diameter of a carbon atom is 3.4 Å. The average separations for those 1,035 with maximum separations >5.0 Å range from 0.14 to 91 Å, with 184 entries having average separations >5.0 Å.) 3,560 entries have maximum separations ≥2.0 Å, and 6,125 ≥ 1.0 Å.
| Range of Separation Averages, Å | Entry Count | Average Resolution, Å |
|---|---|---|
| 0.00-0.20 | 70,134 | 1.65 |
| 0.21-0.50 | 4,446 | 1.64 |
| 0.51-1.00 | 1,358 | 1.66 |
| 1.01-2.50 | 1,128 | 1.86 |
| 2.51-5.00 | 307 | 2.08 |
| 5.01-10.00 | 99 | 2.23 |
| 10.01-20.00 | 39 | 2.16 |
| 20.01-40.00 | 40 | 2.26 |
| ≥ 40.01 | 6 | 2.62 |
Electron Microscopy
| Range of Separation Averages, Å | Entry Count | Average Resolution, Å |
|---|---|---|
| 0.00-0.20 | 1,093 | 3.76 |
| 0.21-0.50 | 33 | 7.13 |
| 0.51-1.00 | 23 | 4.65 |
| 1.01-2.50 | 25 | 3.90 |
| 2.51-5.00 | 9 | 5.42 |
| 5.01-10.00 | 11 | 3.47 |
| 10.01-20.00 | 2 | 8.65 |
| 20.01-40.00 | 2 | 3.71 |
| ≥ 40.01 | 0 |
NMR
Structures determined by NMR typically represent alternate locations or flexibility as multiple models. Only a handful of the ~14,000 NMR entries (May, 2023) in the PDB have AltLoc codes, and these are not used in the conventional manner.
AltLoc Backbone Cases
Case 1: Flexible active site loop broadens range of protection.
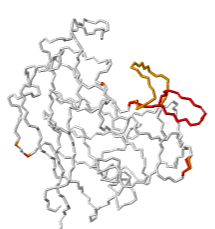
| TRIMCyp protein mediates innate immunity to HIV by inhibiting post-entry retrovirus replication. Evidence suggests that TRIMCyp has evolved through mutations to broaden the range of retroviruses it can inhibit[1]. These mutations render the active site able to assume multiple conformations. In particular, the cyclophilin-binding loop 64-74 (GGNFT HCNGT GG), flanked by double glycine pivots, exhibited flexibility[1]. A crystal structure, 4dgd, resolved two alternate location conformations for the loop residues 66-74, shown at right in red (AltLoc A) and orange (AltLoc B), each at about 50% occupancy.
Explore 4DGD in FirstGlance in Jmol, which has AltLoc visualization/animation capabilities. AltLoc Server Report: |
Case 2: Twist in a potassium channel regulates gating.
|
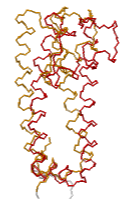
Inwardly rectifying potassium (Kir) channels that pass K+ more easily inwards than outwards have a cytoplasmic regulatory domain. A constriction in the cytoplasmic region of the pore (white, bottom) has been thought to gate potassium channels, but the ion selectivity filter (colored, top) may be equally important in gating. Crystal structures reveal alternative locations: rotation of the cytoplasmic domains relative to the selectivity filter appears to be involved in regulation of selectivity and gating[2][3]. Explore 2WLK in FirstGlance in Jmol, which has AltLoc visualization/animation capabilities. AltLoc Server Report: | 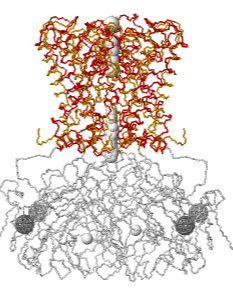
| 
|
| Gated K+ channel 2WLK. Cytoplasmic domains white; transmembrane selectivity filter domains colored: AltLoc A, AltLoc B. | Selectivity filter domain of a single chain of the tetrameric pore. AltLoc A, AltLoc B. |
Additional Cases
- 3PGA, a glutaminase-asparaginase, has a 20-residue flexible loop that is thought to orient substrate and help to transport substrate and product from the active site.
- 3KQU illustrates a helicase ratchet translocation mechanism with its single-stranded DNA in four positions (conformations).
- The asymmetric unit of 4MYD is a trimeric enzyme, in which "two [chains] bind one or both small molecule ligands and the third in the middle is present in two C2-symmetric conformations in a 1:1 ratio without any ligands." Here you will see alternate locations representing the entire middle chain rotated close to 180 degrees.
- 6XKC is a hexamer. Two of the chains are modeled at two different angles relative to the other 4. In animation, these chains wave back and forth.
References
- ↑ 1.0 1.1 Caines ME, Bichel K, Price AJ, McEwan WA, Towers GJ, Willett BJ, Freund SM, James LC. Diverse HIV viruses are targeted by a conformationally dynamic antiviral. Nat Struct Mol Biol. 2012 Mar 11. doi: 10.1038/nsmb.2253. PMID:22407016 doi:10.1038/nsmb.2253
- ↑ Zhou W, Jan L. A twist on potassium channel gating. Cell. 2010 Jun 11;141(6):920-2. PMID:20550927 doi:10.1016/j.cell.2010.05.038
- ↑ Clarke OB, Caputo AT, Hill AP, Vandenberg JI, Smith BJ, Gulbis JM. Domain reorientation and rotation of an intracellular assembly regulate conduction in Kir potassium channels. Cell. 2010 Jun 11;141(6):1018-29. PMID:20564790