Angiotensin-Converting Enzyme (ACE) is both an exopeptidase and endopeptindase first discovered by Skeggs et al. in 1956. [1] ACE is a zinc- and chloride-dependent metallopeptidase that is responsible for the metabolism of key biologically active peptides, namely Angiotensin I and Bradykinin. These two peptides play a critical role in maintaining appropriate blood pressure in the human body along with a host of other homeostatic circulatory functions. See Hypertension & Congestive Heart Failure. ACE catalyzes the conversion of the decapeptide Angiotensin I to the octapeptide Angiotensin II. Due to its critical role in the Renin-Angiotensin-Aldosterone System (RAAS), ACE has been targeted by a number of pharmaceutical compounds to treat hypertension, diabetic nephropathy, and renal failure. [2]
Biological Role
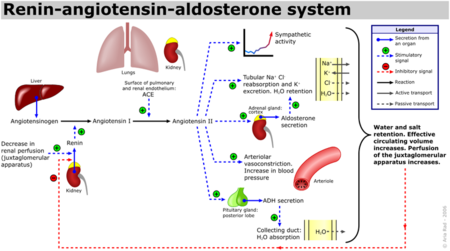
Renin-Angiotensin-Aldosterone System Schematic
ACE is a Zn and Chloride dependent type-1 membrane protein (N-terminal regions are outside the cell). Two types of Angiotensin-converting enzyme exist, ACE1 and ACE2, although the most focus has been on ACE1 which has been attributed with receptor-mediated effects like vasoconstriction, inflammation and cell growth/proliferation. [3] The Renin-Angiotensin System (RAS) is a major regulator of blood pressure in the human body. Renin is an enzyme produced by the liver which cleaves Angiotensinogen into Angiotensin I Angiotensin Ihas the sequence, DRVTIHPFHL, and does not appear to have any biological activity. Angiotensin 1 (See:1n9u) is converted into Angiotensin II (See:1n9v) via the removal of the two C-terminal residues by ACE, yielding the active peptide: DRVTIHPF. [4]
Angiotensin II interacts with two receptor subtypes, AT1 and AT2, which are widely distributed throughout the body. [5] Binding of Angiotensin II to ATI leads to vasoconstriction by vascular smooth muscle cells, resulting in increased blood pressure, as well as the release of fluid and electrolyte homeostasis regulator, aldosterone, by the adrenal glands. Further, Angiotensin II binds to kidney AT1 receptors resulting in sodium ion reabsorption, leading to increased water retention in the blood and subsequent increased blood pressure. [6]
| Selected AT1 and AT2 Receptor-Mediated Effects of Angiotensin II. [7][3]
|
| AT1 Receptor Mediated Effects
| AT2 Receptor Mediated Effects
|
| Vasoconstriction
| Vasodilation
|
| Inflammation
| Inflammation
|
| Cell Growth and Proliferation
| Apoptosis and Fibroblast Growth Inhibition
|
| Cardiovascular effects: Increased Atherogenicity, increased arrhythmogenicity, decreased renal blood flow, increased myocardial contractility.
| Cardiovascular Effects: Improved cardiac function and decreased chornotropic effect.
|
Additionally, Bradykinin, which is inactivated by ACE1, has vasodilatory and cardioprotective properties by promoting the formation of nitric oxide by the endothelium. [8] The essential role ACE1 plays in blood pressure homeostasis is further supported by knockout mice created by Cole et. al. ACE1 knockout mice exhibited an approximate 35% reduction in blood pressure, resulting in hypotension and subsequent organ damage. Thus despite the many systems contributing to blood pressure in mammals, i.e. nitric oxide, endothelin and andregenic stimulation etc. these redundant systems are not enough to overcome a disruption of the RAAS. [9] It should be noted that AT2 binding of Angiotensin II results in many processes that counterbalance the binding of AT1. See the schematic image of the Renin-Angiotensin-Aldosterone System at the left for a visual description and the table below for selected Angiotensin receptor-mediated effects of binding Angiotensin II.
Structural Analysis, Mechanism, & Activation
Structure of ACE1
The larger, somatic form of ACE1 has two metalloproteinase domains (N- and C-terminal domains), each containing the canonical Zn binding motif, HEXXH. Despite their similar structures and protease activity, only the C-terminal domain is critical for blood pressure regulation.[10] The smaller, testis-specific form of ACE1 (tACE) only contains the C-terminal metalloproteinase domain (identical to that of somatic ACE1), along with a hydrophobic membrane-anchoring domain and a small highly glycosylated N-terminal region. [5] The structure of tACE adopts a predominantly helical ellipsoid structure with a , dividing the protein into two subdomains, S1 (Green) and SII (Purple). The boundaries of the groove are formed by helices 13, 14, 15, and 17 as well as beta strand 4. On top of the groove a , preventing bulky ligands from accessing the active site and adding to ACE1’s specificity. [11]
Zinc Coordinated Substrate Binding and Catalytic Mechanism
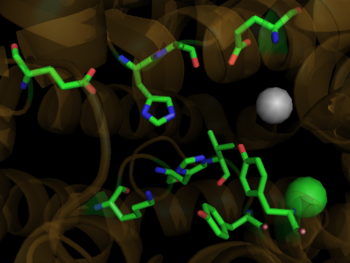
Binding Site of ACE with Zn Ion in Grey and Cl Ion in Green
1o86Zinc is a critical component of the ACE1 catalytic binding site. Helix 13 contains , utilizing His 383 and His 387 along with Glu 411 on helix 14. The active site of ACE1 incorporates the .[11] Incoming substrate binds zinc by displacing the zinc bound water molecule. The water molecule subsequently binds the nearby Glu 384 resulting in polarization between the negative glutamate carboxylate group and the positive zinc ion. [12]This enhances the nucleophilicity of the water oxygen, promoting attack on the substrate peptide carbonyl carbon. The proton accepted by the active site glutamate is shuttled to the nitrogen, possibly forming a tetrahedral gem-diolate intermediate with the help of Tyr 523. The dipeptide product formed from the cleavage of the C-N bond is released in the protonated form. [11]The remaining peptide substrate is stabilized via hydrogen bond interactions between Ala 354 and the new terminal amide, His 353 & His 513 with the secondary carbonyl group, and Tyr 520 and Lys 511 and the terminal carboxylate. [6]
Chloride Activation
The C-Domain active site is strongly activated by chloride ion.[13] Two buried chloride ions are found in the ACE1 crystal structure. The first is about 21 angstroms from the zinc ion and isand is surrounded by a hydrophobic shell of four tryptophans. The second chloride ion is located . It is believed that the first chloride ion stabilizes the active ACE1 structure. The primary ligand for the second chloride ion, both of which are found in the active site. [11]
Medical Implications
Medical Implications and Inhibitor Binding
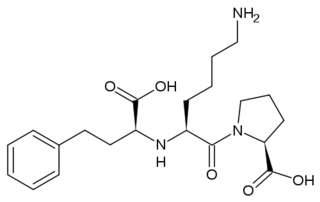
The ACE Inhibitor, Lisinopril
Several studies have validated a pathological role for Angiotensin II in cardiac, renal and vascular diseases like hypertension and diabetic renal failure. [3] The increased blood pressure and oxidative stress associated with elevated levels of Angiotensin II can result in endothelial dysfunction and microvascular damage, ultimately leading to heart failure, stroke and kidney disease among other clinical manifestations. [7] Bradykinin, a small peptide that counterbalance the effects of Angiotensin II by acting as a strong vasodilator upon binding AT2, is degraded by the same ACE1 enzymes which create Angiotensin II from Angiotensin I. Since ACE1 is the primary producer of Angiotensin II and primary degrader of Bradykinins, the development of ACE1 inhibitors has been a major focus for drug developers looking to fight these cardiovascular and renal conditions. [7] ACE1 inhibitors like Captopril (1uzf, Capoten), Ramipril (Altace), Lisinopril, (1o86, Perindopril, Prinivil, ACE Inhibitor Prinivil, ACE Inhibitor Lisinopril), and Benazepril (Lotensin) have proven to be effective at reducing Angiotensin II based pathologies. Sale of ACE1 inhibitors topped $5 billion in 2009 with over 150 million prescriptions filled.[14]
Crystal structures of ACE1 with bound competitive inhibitors reveal the mechanism of inhibition. Lisinopril binds to the ACE1 binding site in an extended conformation, with its phenyl group oriented toward the active site lid while the lysine chain parallels the zinc binding motif helix. [11] Lisinopril makes a , utilizing His 353, Ala 354 (backbone oxygen), Glue 384, Lys 511, His 513, Tyr 520, Tyr 523 and Glu 162 as well as van der Waals interactions between the phenylpropyl group and Val 518. [11]. Another inhibitor, Captopril, , forming electrostatic interactions with His 353, Glu 384, Lys 511, His 513 and Tyr 520, along with zinc cation. Enalaprilat, a third competitive inhibitor (1uze), with His 353, Ala 354 (Backbone oxygen), Glue 384, Lys 511, His 513, Tyr 520 and Tyr 523 along with the zinc cation. All three inhibitors are very effective and are FDA approved for treatment of Angiotensin II related hypertension and other cardiovascular and renal disorders. [15] Other ACE Inhibitors approved by the FDA include Ramipril, Benazepril, Perindopril, Trandolapril, Enalapril (Vasotec) and Trandolapril
See
Treatments:ACE Inhibitor Pharmacokinetics References
ACE Inhibitor Pharmacokinetics
Hypertension & Congestive Heart Failure
Lisinopril-Angiotensin Converting Enzyme
ACE2 (Hebrew)
Treatments:Hypertension.
ACE2 and coronavirus (SARS-CoV and COVID-19) entry into the cell
During the SARS scare of 2002-2003, extensive research was focused on the interactions between the SARS virus and its host cells. It was determined that the severe acute respiratory syndrome conavirus (SARS-CoV) enters cells through the activities of a spike shaped protein on its outer envelope. [16] The Receptor Binding Domain (RBD) of SARS-CoV binds to ACE2, on the surface of the cell. It was determined that by changing a few selected residues on either the SARS-CoV RBD or the ACE2 binding site, the virus becomes significantly more infectious. (3d0g), namely at residues 31, 35, 38, & 353 in ACE2 or residues 479 and 487 in the SARS-CoV RBD, are what allowed for SARS transmission from Civets to Humans. In fact, in those SARS strains which were determined to be most infectious, the unfavorable electrostatic interactions at the binding interface were removed via mutations at the critical residues 479 and 487. [16]
In 2020 Zhou et al. (Nature. 2020; 579: 270-273) and Hoffmann et al. (Cell. 2020; 181: 271-280) showed that SARS-CoV-2, the COVID-19 coronavirus causing the global 2019-2020 pandemia, uses ACE2 as a receptor protein to enter and infect cells, just as SARS-CoV does. Cell entry requires the binding of the S1 region of the virus spike (S) protein to ACE2 followed by the fusion of the viral and cellular membranes produced by the S2 subunit of the S protein. Beforehand, this process requires priming of the S protein by host cell proteases, which is performed by TMPRSS2 and the endosomal cysteine proteases cathepsin B and L (CatB/L). These results suggest therapeutic targets for COVID-19. One is targeting the binding interface between SARS-2-S protein and ACE2, and the other is to inhibit the serine protease activity of the proteases responsible for SARS-2-S protein priming.
3D Structures of Angiotensin-Converting Enzyme
Angiotensin-Converting Enzyme 3D structures



