Ken Engle SANDBOX
From Proteopedia
The Enzyme Pyruvate Decarboxylase
| |||||||||
| 1zpd, resolution 1.86Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Pyruvate decarboxylase, with EC number 4.1.1.1 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
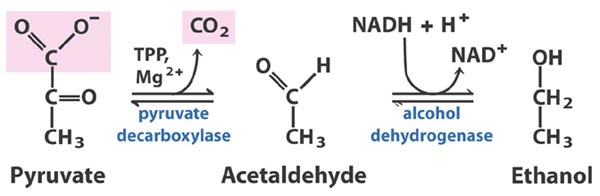
Image 1: Reaction catalyzed by pyruvate decarboxylase: pyruvate + thiamine pyrophasphate (TPP) → hydroxyethyl-TPP + CO2.
Importance in Anaerobic Metabolism
Pyruvate, NADH, and ATP are the products of glycolysis. Under anaerobic conditions, pyruvate undergoes fermentation to oxidize NADH to NAD+, so glycolysis can continue. In alcoholic fermentation, which occurs in some yeast, this is a two-step process. The first involves the Enzyme pyruvate decarboxylase (PDC). The pyruvate is decarboxylated to an acetaldehyde. This acetaldehyde then undergoes a reaction catalyzed by alcohol dehydrogenase to produce ethanol; this is the step in which the NAD+ is restored [1].
Structure
Pyruvate decarboxylase is a homotetramer. Each identical subunit consists of approximately alternating α-helices and β-sheets, and 2 domains exist within each 60kDa . This means its SCOP category is alpha and beta protein [2]. Being a homotetramer, pyruvate PDC has 4 identical that are green surrounding the ligands when the previous link is selected.
Active Site
The active site of PDC in Zymomonas mobilis consists of Glu50, Glu 473, Asp27, and His114 [3]. Hydrogen bonding occurs between the substrate and Asp27, His114, and Thr72. Yeast active site residues are similar. In the catalytic step of the reaction mechanism, , shown in red, donates a proton to the pyruvate. The scene shows the close proximity of this residue to the pyruvate. The negative charge of the Glu residue following the protonation of the substrate leads to the destabilization of the pyruvate carboxylate group. Next the carboxyl group leaves. Following decarboxylation in the final step of the mechanism, release of acetaldehyde, a proton is transferred to the Glu473 residue from a cofactor. After the protonation in a concerted step, a water molecule donates a proton to the substrate while receiving a proton from Glu473. As the proton is taken from the substrate, the electrons move to form a carbonyl, which leads to the release of the acetaldehyde[4].
Regulation
PDC is regulated by substrate activation. This means that if substrate is not present in the pathway, the protein will be "off." The residue that is bound to start a cascade of events resulting in the activation of the enzyme is [5] which is highlighted in pink in the scene. This process allows the enzyme to be on when its function is necessary and off when it would not be catalyzing the reaction even if it were on.
ThDP an Important Cofactor
Thiamine diphosphate (ThDP) is an important cofactor in the pyruvate, acetaldehyde reaction. The of this cofactor can be seen in this scene near the four active sites though they are hidden in other scenes by the ligand, pyruvate. ThDP actually binds the substrate during the first step of the reaction at C2 of the pyruvate. It is this ThDP that changes the environment of the active site which leads to the protonation or deprotonation of Glu473. When ThDP is not bound, the active site is not even open to bind pyruvate. When it binds, it causes a conformational change, moving Glu473 in such a way that forms a pocket for pyruvate’s methyl group[6].
References
- ↑ Garrett, R.H., & Grisham, C.M. (2007). Biochemistry. Belmont, CA: Thompson.
- ↑ Dobritzsch D, Konig S, Schneider G, Lu G. High resolution crystal structure of pyruvate decarboxylase from Zymomonas mobilis. Implications for substrate activation in pyruvate decarboxylases. J Biol Chem. 1998 Aug 7;273(32):20196-204. PMID:9685367
- ↑ Pei XY, Erixon KM, Luisi BF, Leeper FJ. Structural Insights into the Prereaction State of Pyruvate Decarboxylase from Zymomonas mobilis . Biochemistry. 2010 Feb 5. PMID:20099870 doi:10.1021/bi901864j
- ↑ Pei XY, Erixon KM, Luisi BF, Leeper FJ. Structural Insights into the Prereaction State of Pyruvate Decarboxylase from Zymomonas mobilis . Biochemistry. 2010 Feb 5. PMID:20099870 doi:10.1021/bi901864j
- ↑ Sergienko EA, Jordan F. Catalytic acid-base groups in yeast pyruvate decarboxylase. 3. A steady-state kinetic model consistent with the behavior of both wild-type and variant enzymes at all relevant pH values. Biochemistry. 2001 Jun 26;40(25):7382-403. PMID:11412092
- ↑ Pei XY, Erixon KM, Luisi BF, Leeper FJ. Structural Insights into the Prereaction State of Pyruvate Decarboxylase from Zymomonas mobilis . Biochemistry. 2010 Feb 5. PMID:20099870 doi:10.1021/bi901864j