Matrix Metalloproteinase 12
From Proteopedia
| |||||||||
| Human MMP12 complex with Ca+2 and Zn+2 ions, 2oxu | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | CA, ZN | ||||||||
| Gene: | MMP12, HME (Homo sapiens) | ||||||||
| Activity: | Macrophage elastase, with EC number 3.4.24.65 | ||||||||
| Related: | 1y93, 1rmz, 2oxw, 2oxz, 2oy2, 2oy4 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Matrix Metalloproteinase 12 is a human protease which is involved in tissue remodelling and cell signalling. The MMPs are secreted as inactive proproteins and then activated after a cleavage by extracellular proteinases.
MMPs are sorted into 17 classes depending on their localization in the cell, and on their substrates. For instance, MMP12 is a macrophage metalloelastane coded by the MMP12 gene.
In healthy cells, 2oxu is implied in tissue remodelling and cell signalling. Its role is to allow the migration of the macrophages in tissues by hydrolysing soluble and insoluble elastin of the extracellular matrix by involving two cofactors: zinc and calcium. Zn2+ atoms are part of the enzyme whereas Ca2+ atoms are hydrogen bounded to the backbone of the protein.
Because of its activity, this protein is also involved in metastasis and tumor development; that's why it is considered as an important target for drug therapies.
Contents |
From structural data to the enzymatic mecanism
Thanks to X-ray crystallography, the structure of 2oxu has been solved with 1,24 Å resolution. This enzyme consists of four alpha helixes and five beta sheets organized like as you can see on the J-mol figure.
The hydrolysis of the Substrate can be divided in four essential events:
1- Substrate's fixation
2- Substrate's cleavage
3- First fragment's release
4- Second fragment's release
The substrate polypeptide sequence which is recognized by the enzyme is the following one : ProGlnGly(206)IleAlaGly(209). It is known to be cleaved between Gly(206) and ILE. Before the addition of the substrate, the active site is closed.
A video of this hydrolysis is available online
Substrate's fixation
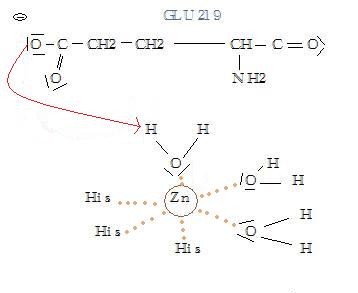
The active site of the enzyme is composed by one Glu residue near 3 His residues which maintain a Zn2+ atom coordinated with three water molecules. One of them is as well bound to the Glu residue thanks to a hydrogen bond. The second Zn2+ atom is not involved in the active site. At first, the Gly 206 residue of the substrate binds the active site thanks to the Zn2+ atom. When it binds it takes the place of unstable water molecules and establishes stabilizing interactions with the active site thanks to its C terminal part. Then, the Ala 182 residue of the enzyme makes a hydrogen bond with the NH group of the substrate: this allows the substrate to enter the cavity of the catalytic site. The rest of the protein is stabilized by 4 hydrogen bonds with the amino acid located in the cavity.
Substrate's cleavage
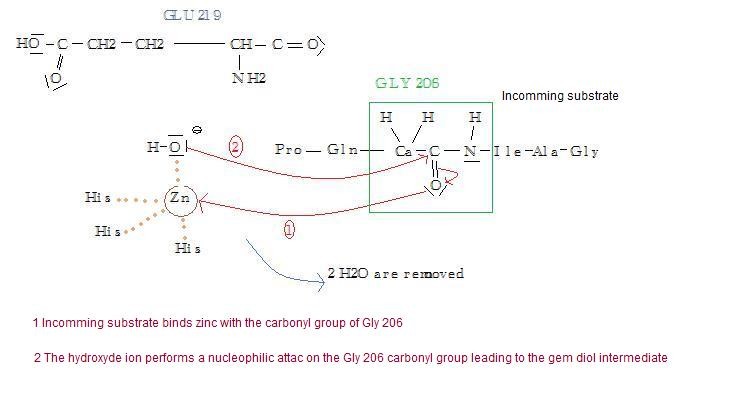
A proton of one of the three H2O moves to the Glu 219 catalytic site's residue. It creates a hydroxyl group with a high nucleophilic character. That leads to a nucleophilic attack by zinc-coordinated hydroxyl group, on one of the carbonyl group of the substrate (Gly206). It leads to what is called a gem-diol intermediate form of the substrate. Two fragments are then sitting in the cavity: IleAlaGly209 and ProGlnGly206.
First fragment's release
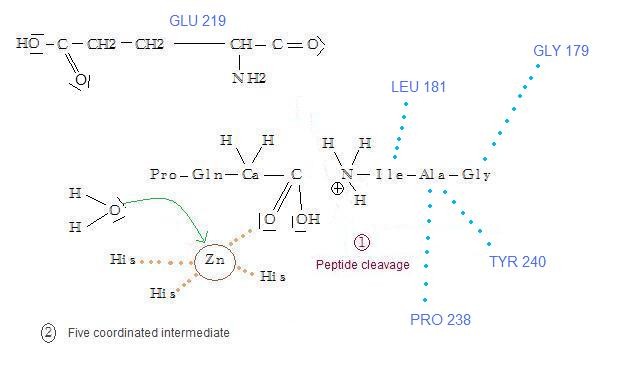
The fragment IleAlaGly is strongly bounded to the enzyme thanks to the bonds that have been created at the fixation's step. But the fragment ProGlnGly just remains in place thanks to a Gly206 monodentate coordination with the Zn atom. Then a water molecule binds this Zn atom: it becomes five coordinated, which facilitates the detachment of the ProGlnGly fragment through an associative ligand exchange mechanism.
Second fragment's release
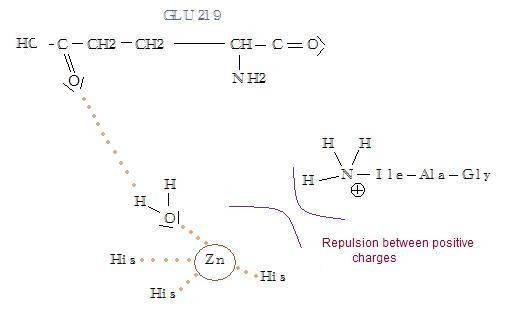
IleAlaGly remains in the active site but is subjected to a rearrangement. After the release of ProGlnGly, repulsion between positively charged zinc ion and NH3+ (coming from the peptide's cleavage) increases. It forces IlaAlaGly to enter deeper in the substrate's cavity to make hydrophobic interactions. The transfer of this fragment prevents Ala208 to continue binding Pro208. The former bound is broken, and the fragment is no more stabilized. Finally the cavity opens and the fragment is released.
Regulation
One of the two Zn2+ ions is involved in the catalytic site to catalyse the reaction. The second Zn2+ atom and the three Ca2+ atoms bind the enzyme to stabilize its 3D structure. Thus Ca2+ concentrational changes may regulate MMP12 activity by destabilizing the structure of the enzyme.
An other way to lower the enzymatic activity is to modify some residues of the enzyme. The main question is : what for residues ? As we said before, several ionzable residues are found in the catalytic site. These ionizable residues allow electrostatic interractions between the enzyme and the substrate, and that decrease the activation energy of the reaction. If these residues are modified, the activation energy increases and the reaction is done at a lower rate. Let's look at some example in the case of 2oxu.
- Glu 219 plays a very important role in the substrate's fixation : if it is mutated and replaced by an non-ionizable residue, it lowers the enzymatic activity.
- If the ionization of NH2 to NH3+ can be prevented, it won't lead to any charge repulsion with Zn2+ : the substrate won't enter the cavity very deeply and so will remain bound to the enzyme, preventing the next binding with the next substrate.
Research for inhibitors : current stakes
MMPs are thought to play a major role on the cellular behaviour such as cell proliferation, allowing a tumor to spread for instance. That's the reason why researchers are still working on this molecule : to learn more about its structure, in order to find new ways to inhibit it. Some natural inhibitors have already been found : they are called Tissue Inhibitor of MetalloProteinases (TIMPs) and are sorted into four classes : TIMP-1, TIMP-2, TIMP-3 and TIMP-4. Furthermore, researchers have successfully produced synthetic inhibitors : These inhibitors contain a chelating group, which binds the catalytic zinc atom involved in the catalytic site. Common chelating groups include hydroxamates, carboxylates, thiols, and phosphinyls. Hydroxymates are particularly potent inhibitors of MMPs thanks to their bidentate chelation of the zinc atom. A 2oxu inhibitor can also inhibit other MMPs after a few modifications : Only the substituents interacting with the pocket have to be modified because of the various binding pockets of the MMPs. That means that from a a single inhibitor, researchers are able to create more or less specificity toward several enzymes.
3D structures of matrix metalloproteinase
References
Websites :
- PDB A Resource for Studying Biological Macromolecules [1]
- National Library of Medicine - Medical Subject Headings [2]
- Les metalloprotéases matricielles [3]
Publications :
- Bertini I, Calderone V, Fragai M, Luchinat C, Maletta M, Yeo KJ. Snapshots of the reaction mechanism of matrix metalloproteinases. Angew Chem Int Ed Engl. 2006 Dec 4;45(47):7952-5. PMID:17096442 doi:10.1002/anie.200603100
- Gossas T, Danielson UH. Characterization of Ca2+ interactions with matrix metallopeptidase-12: implications for matrix metallopeptidase regulation. Biochem J. 2006 Sep 15;398(3):393-8. PMID:16737445 doi:10.1042/BJ20051933