Matrix metalloproteinase
From Proteopedia
FunctionMatrix metalloproteinases (MMP) are Zinc-dependent endopeptidases. MMP degrades extracellular matrix proteins. They are inhibited by proteases called tissue inhibitors of metalloproteinase (TIMP). The pro-MMP contains a pro-peptide which must be removed to render the MMP active[1]. See details in MMPs are produced by 28 different genes and are classified according to their protein substrates.
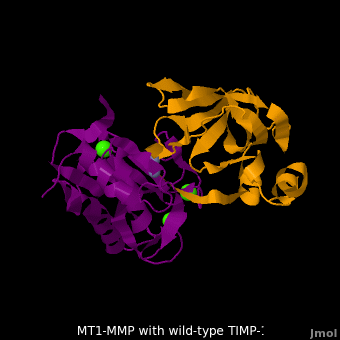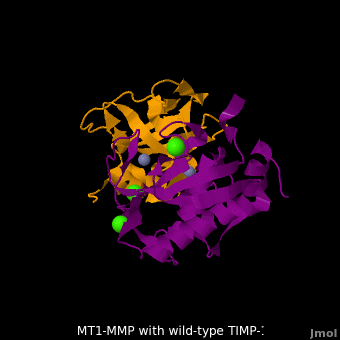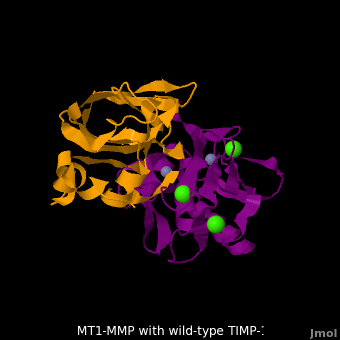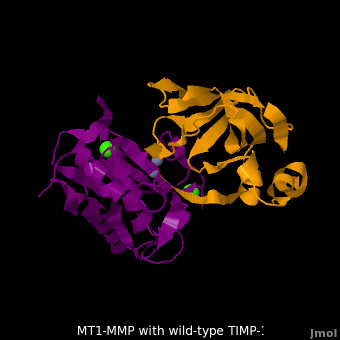
RelevanceMMPs have a role in cancer progression[2]. MMP-2 and MMP-9 secretion is elevated in ovarian cancer and are associated with poor prognosis[3]. MMP-8, MMP-9, MMP-13 and MMP-14 have a role in periodontal diseases[4]. MT1-MMP-TIMP-1 complexThe human matrix metalloproteinases (MMPs) family comprises a large group of structurally homologous zinc-dependent endopeptidases (e.g. membrane type-1 matrix metalloproteinase (MT1-MMP) (darkmagenta) and membrane type-3 matrix metalloproteinase (MT3-MMP) (magenta), click to see structural comparison) that perform a wide variety of biological roles. In general, the MMPs are inhibited unselectively by all four known tissue inhibitors of metalloproteinases (TIMPs 1-4) which have 40-50% sequence identity. For example, membrane type-3 matrix metalloproteinase (MT3-MMP) can form complex with wild-type TIMP-1 (1uea, colored orange). The WT-TIMP-1 binding interface (cyan) is mainly composed of the N-terminal segment that approaches the active site, the AB loop (Thr33-Tyr35), the CD loop (Ala65-Cys70), and the EF loop (Thr97-Ser100). The pivotal residue, threonine 98 (Thr98), is shown as red sticks. In general, five main chain hydrogen bonds (Cys1-Ser68, Val69-Met66, Gly71-Met66, Cys70-Glu67, and Cys70-Thr98) are intimately involved in the conformational stability of TIMP binding interface when bound to MMP. Membrane type-1 matrix metalloproteinase (MT1-MMP) (darkmagenta) also forms complex with wild-type TIMP-1 (2j0t, colored orange), producing similar hydrogen bond network in the WT TIMP-1 binding interface as well as in the case with MT3-MMP. This network of hydrogen bonds stabilizes the CD and EF loops that compose the binding interface. Importantly, the hydrogen bond between Cys1 and Ser68 may position the amino and carboxyl groups of Cys1 to effectively coordinate the Zn2+ ion. However, this MT1-MMP-WT-TIMP-1 complex is not tight-binding. MT1-MMP is unique since even though it exhibits high structural homology to all MMPs, it is not inhibited by TIMP-1, but is inhibited by the structural homologous TIMP-2 (1bqq). The single point mutation T98L (mutant TIMP-1 is colored in yellow with T98L shown in red) transformed TIMP-1 into a high affinity inhibitor of MT1-MMP (3ma2). WT-TIMP-1, WT-TIMP-2, and TIMP-1 T98L mutant have kinetic dissociation binding constant (KD) 1.53 x 10-6, 5.61 x 10-8, and 8.70 x 10-8, respectively. So, KD of WT-TIMP-2 is 2 orders of magnitude smaller than that of WT-TIMP-1, indicating the weak affinity between MT1-MMP and WT-TIMP-1. The TIMP-1 T98L mutant regained high-affinity binding to MT1-MMP, resulting in a 2 order of magnitude decrease in KD, similar to the case for WT-TIMP-2, the in vivo inhibitor of MT1-MMP. The overall structures of the complexes of MT1-MMP-WT-TIMP-1 and MT1-MMP-mutant-T98L-TIMP-1 are relatively similar. Even the structure of MT3-MMP-WT-TIMP-1 is similar to those of MT1-MMP-TIMP-1s (with wild-type and TIMP-1 T98L mutant). Leu98 is pointing toward MT1-MMP residues Pro259 and Phe260, establishing a strong hydrophobic core, which is situated near the MT1-MMP catalytic Zn2+ ion surrounded by His239, His243, and His249. So, this T98L replacement may stabilize the entire area by establishing a strong hydrophobic core upon binding to the enzyme. However, it seems unlikely that these additional bonds could account for the entire binding effect between MT1-MMP and TIMP-1. Statistical analysis of the key hydrogen bond stabilities in the TIMP-1 T98L mutant reveals that the hydrogen bonds network in mutant form is significantly more stable than that in WT-TIMP-1. Mutations that enhance hydrogen bond stability contribute to the stability of the bound-like, less flexible, conformation of TIMP-1, which eventually results in increasing binding affinity for MT1-MMP. Thus, mutation affected the instrinsic dynamics of the inhibitor rather than its structure, thereby facilitating the interaction [5]. 3D structures of matrix metalloproteinaseMatrix metalloproteinase 3D structures
|
| |||||||||||
References
- ↑ Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999 Jul 30;274(31):21491-4. PMID:10419448
- ↑ Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011 Jan;278(1):16-27. doi: 10.1111/j.1742-4658.2010.07919.x. Epub 2010, Nov 19. PMID:21087457 doi:http://dx.doi.org/10.1111/j.1742-4658.2010.07919.x
- ↑ Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009 May;21(5):1323-33. PMID:19360311
- ↑ Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993 May;64(5 Suppl):474-84. PMID:8315570 doi:http://dx.doi.org/10.1902/jop.1993.64.5s.474
- ↑ Grossman M, Tworowski D, Dym O, Lee MH, Levy Y, Murphy G, Sagi I. Intrinsic protein flexibility of endogenous protease inhibitor TIMP-1 controls its binding interface and effects its function. Biochemistry. 2010 Jun 14. PMID:20545310 doi:10.1021/bi902141x