X-ray crystallography
From Proteopedia
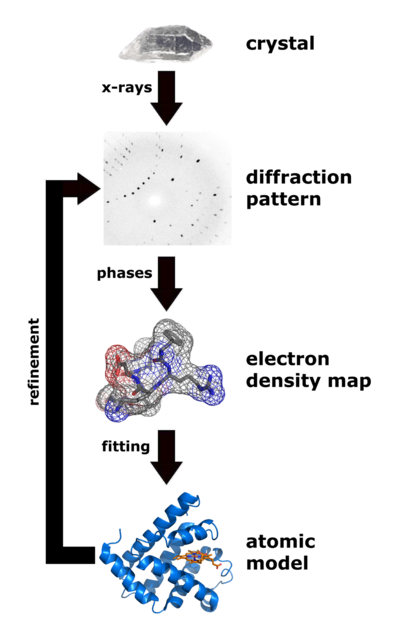
|
| Flow chart showing the major steps in X-ray protein crystallography. (Image from Wikimedia courtesy Thomas Splettstoesser.) |
About 88% of the models (entries) in the World Wide Protein Data Bank were determined by X-ray crystallography (August, 2021). (About 7% were determined by solution nuclear magnetic resonance, and about 5% by cryo-EM.) Analysis of x-ray diffraction patterns from protein crystals produces an electron density map, into which an atomic model of the protein is fitted. Major errors sometimes occur when fitting models in to low-resolution electron density maps (see Quality assessment for molecular models). The value of Free R is the best clue as to whether major errors may be present in a published model.
Obtaining diffraction-quality crystals of proteins can be very difficult for some proteins, despite many recent advances[1]. For every new protein sequence targeted for X-ray crystallography, about one in twenty is solved[2][3]. Efforts are underway to improve this success rate[4].
 | Crystals of the flagellar hook protein FlgE from C. jejuni produced in the Samatey lab. |
Publication of solved structures involves depositing an atomic coordinate file (PDB file) in the World Wide Protein Data Bank.
About two-thirds of published crystallographic models can be improved by automated re-refinement: see PDB REDO.
Contents |
See Also
- Methods for Determining Atomic Structures
- Electron density maps
- X-ray Crystallography at Wikipedia
- Protein Crystal Gallery
- Crystal contacts
- Biological Unit
- Quality assessment for molecular models
- Resolution
- R value
- Free R
- Hydrogen in macromolecular models
- Water in macromolecular models
DNA X-Ray Diffraction
- Links to explanations of Rosalind Franklin's original DNA diffraction pattern, used by Watson and Crick, will be found in the See Also section of the article on DNA.
Further Reading
- Crystallography Made Crystal Clear: a guide for users of macromolecular models, a book by Gale Rhodes.
- Introduction to PDB Data.
Notes & References
- ↑ McPherson A, Gavira JA. Introduction to protein crystallization. Acta Crystallogr F Struct Biol Commun. 2014 Jan;70(Pt 1):2-20. doi:, 10.1107/S2053230X13033141. Epub 2013 Dec 24. PMID:24419610 doi:http://dx.doi.org/10.1107/S2053230X13033141
- ↑ Success Rates in Protein Crystallography
- ↑ Structural Genomics Progress Chart
- ↑ Boutet S, Lomb L, Williams GJ, Barends TR, Aquila A, Doak RB, Weierstall U, Deponte DP, Steinbrener J, Shoeman RL, Messerschmidt M, Barty A, White TA, Kassemeyer S, Kirian RA, Seibert MM, Montanez PA, Kenney C, Herbst R, Hart P, Pines J, Haller G, Gruner SM, Philipp HT, Tate MW, Hromalik M, Koerner LJ, van Bakel N, Morse J, Ghonsalves W, Arnlund D, Bogan MJ, Caleman C, Fromme R, Hampton CY, Hunter MS, Johansson L, Katona G, Kupitz C, Liang M, Martin AV, Nass K, Redecke L, Stellato F, Timneanu N, Wang D, Zatsepin NA, Schafer D, Defever J, Neutze R, Fromme P, Spence JC, Chapman HN, Schlichting I. High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography. Science. 2012 May 31. PMID:22653729 doi:10.1126/science.1217737
