We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Herceptin - Mechanism of Action
From Proteopedia
(Redirected from HER2)
| |||||||||||
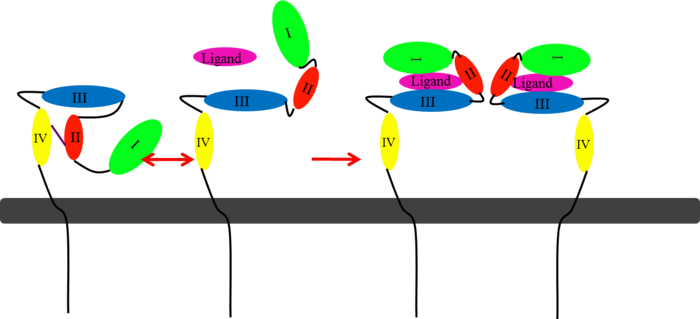
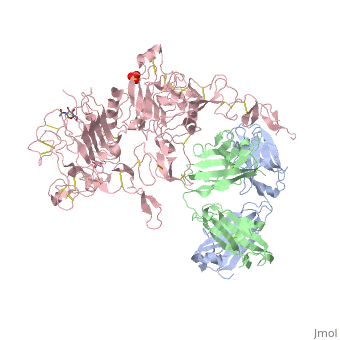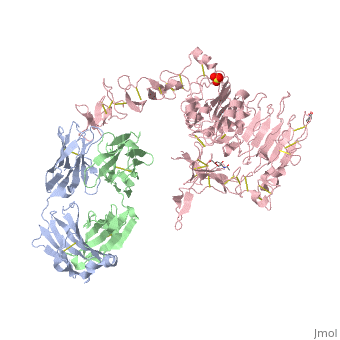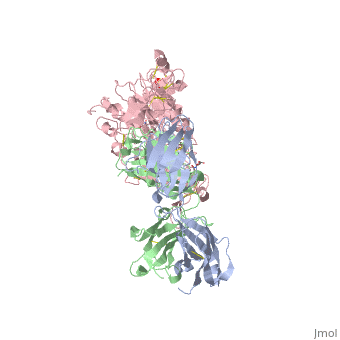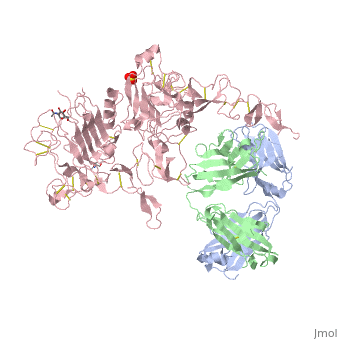
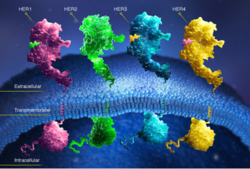
(Figure 6) This picture illustrates the mechanism in which EGFR, HER3, and HER4 change conformation in order to dimerize and activate further cell signaling. A) Sub-domain I (green) is sepearated from sub-domain III (blue). Sub-domain II (red) forms an interaction (purple line) with sub-domain IV (yellow). This conformation allows sub-domain II to be hidden and unavailable for dimerization. B) The interaction between sub-domain II and sub-domain IV can be temporarily broken allowing for the receptor to become more available for ligand-binding. C) Upon ligand-binding, if the interaction of sub-domain II and IV is still formed, the interaction between sub-domain II and sub-domain IV is broken and allows sub-domain II to become available for homo- or hetero-dimerization with another receptor from the HER family[12].
References
- ↑ "What Are the Key Statistics about Breast Cancer?" Breast Cancer. Cancer.org, 31 Oct. 2012. Web. Nov. 2012. <http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics>.
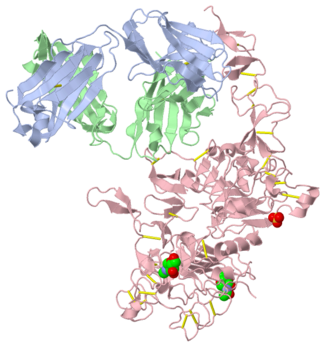
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Cho, Hyun-Soo, Karen Mason, Kasra X. Ramyar, Ann Marie Stanley, Sandra B. Gabelli, Dan W. Denney, Jr., and Daniel J. Leahy. "Structure of the Extracellular Region of HER2 Alone and in Complex with the Herceptin Fab." Letters to Nature 421 (2003): 756-60. PubMed.gov. Web. Oct. 2012.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 "HER2 Dimerization: A Key Component of Oncogenic Signaling in HER2 Breast Cancer." HER2+ Breast Cancer. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/hdis/her2-dimerization/index.html>.
- ↑ "Herceptin Development Timeline." Genentech: Medicines. Genentech, n.d. Web. Nov. 2012. <http://www.gene.com/gene/products/information/oncology/herceptin/timeline.html>.
- ↑ 5.0 5.1 Bazley, L. A., and W. J. Gullick. "The Epidermal Growth Factor Receptor Family." Endocrine-Related Cancer. Society for Endocrinology and European Society of Endocrinology, 2005. Web. Oct. 2012. <http://erc.endocrinology-journals.org/content/12/Supplement_1/S17.full>.
- ↑ 6.0 6.1 Satyanarayanajois, Seetharama, Stephanie Villalba, Liu Jianchao, and Go Mei Lin. "Design, Synthesis, and Docking Studies of Peptidomimetics." Chem. Biol. Drug Des. 74.3 (2009): 246-57. National Institute of Health. Web. Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2866155/pdf/nihms-190276.pdf>.
- ↑ Banappagari, Sashikanth, Sharon Ronald, and Seetharama Satyanarayanajois. "Structure-activity Relationship of Conformationally Constrained." Medchemcomm. 2.8 (2011): 752-59. National Institute of Health. Web. Oct. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3163471/pdf/nihms308284.pdf>.
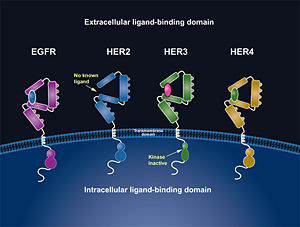
- ↑ 8.0 8.1 8.2 8.3 "HER Pathways Are of Critical Importance in Cancer." HER Receptors Overview. Genentech, n.d. Web. 09 Nov. 2012. <http://www.biooncology.com/research-education/her/overview/index.html>.
- ↑ Ryzhkov, Andrew. "Trastuzumab." Wikipedia. Wikimedia Foundation, 11 Aug. 2012. Web. 09 Nov. 2012. <http://en.wikipedia.org/wiki/Trastuzumab>.
- ↑ 10.0 10.1 Jiang, Beihai, Wenbin Liu, Hong Qu, Lin Meng, Shumei Song, Tao Ouyang, and Chengchao Shou. "A Novel Peptide Isolated from a Phage Display Peptide Library with." The Journal of Biological Chemistry 280.6 (2005): 4656-662. The Journal of Biological Chemistry. Web. Oct. 2012. <http://www.jbc.org/content/280/6/4656.full.pdf+html>.
- ↑ 11.0 11.1 "ERBB2." Genetics Home Reference. U.S. National Library of Medicine, 5 Nov. 2012. Web. 09 Nov. 2012. <http://ghr.nlm.nih.gov/gene/ERBB2>.
- ↑ 12.0 12.1 12.2 Lammerts Van Bueren, Jeroen, Wim K. Bleeker, Annika Bra¨ Nnstro¨m, Anne Von Euler, Magnus Jansson, Matthias Peipp, Tanja Schneider-Merck, Thomas Valerius, Jan G. J. Van De Winkel, and Paul W. Parren. "The Antibody Zalutumumab Inhibits Epidermal Growth." PNAS 105.16 (2008): 6109-114. Web. Nov. 2012. <http://www.pnas.org/content/105/16/6109/F1.expansion.html>.
- ↑ Saxon, Marian L., and David C. Lee. "Mutagenesis Reveals a Role for Epidermal Growth Factor Receptor Extracellular Subdomain IV in Ligand Binding." The Journal of Biological Chemistry 274.40 (1999): 28356-8362. PubMed.gov. Web. Oct. 2012. <http://www.jbc.org/content/274/40/28356.long>.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 Gajria, Devika, and Sarat Chandarlapaty. HER2-amplified Breast Cancer: Mechanisms of Trastuzumab 11.2 (2011): 263-75. National Institute of Health. Web. Oct. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3092522/pdf/nihms289124.pdf>.
- ↑ Wang Z, Cheng L, Guo G, Cheng B, Hu S, Zhang H, Zhu Z, Niu L. Structural insight into a matured humanized monoclonal antibody HuA21 against HER2-overexpressing cancer cells. Acta Crystallogr D Struct Biol. 2019 Jun 1;75(Pt 6):554-563. doi:, 10.1107/S2059798319006995. Epub 2019 May 31. PMID:31205018 doi:http://dx.doi.org/10.1107/S2059798319006995