Image:Zinc-pong.gif
From Proteopedia
No higher resolution available.
Zinc-pong.gif (300 × 256 pixel, file size: 980 KB, MIME type: image/gif)
Summary
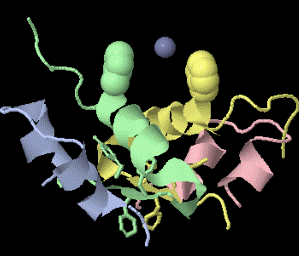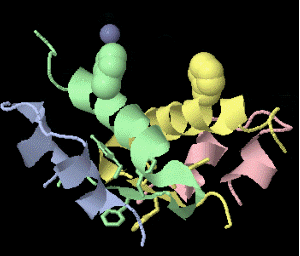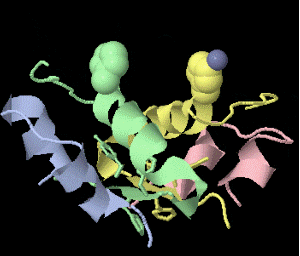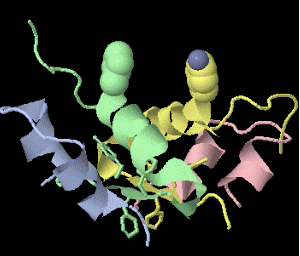
Strange artifact when pymol made a morph between insulin in the T and the R state. Insulin forms a trimer of dimers, with two zinc atoms on the three-fold axis bound to histidines. Apparently, chain IDs of the zinc atoms were inconsistent between files, so the zinc atom is shown switching ligands. It looks like ping pong, so I called it zinc pong.
Licensing
Creative Commons Attribution-Share Alike 3.0 License
![]()
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 21:48, 13 July 2019 | Karsten Theis (Talk | contribs) | 300×256 | 980 KB | Strange artifact when pymol made a morph between insulin in the T and the R state. Insulin forms a trimer of dimers, with two zinc atoms on the three-fold axis bound to histidines. Apparently, chain IDs of the zinc atoms were inconsistent between files, s |
- Edit this file using an external application
See the setup instructions for more information.
Links
There are no pages that link to this file.
