Methylenetetrahydrofolate reductase
From Proteopedia
Methylenetetrahydrofolate reductase (MTHFR) or 5,10-methylenetetrahydrofolate reductase is an enzyme of folate one-carbon metabolism. The enzyme is present in both eukaryotes and prokaryotes however, the structure is unique in eukaryotes as it has a regulatory domain that binds to S-adenosylmethionine (SAM). The enzyme has an essential role in SAM biosynthesis in order to promote homeostasis within the folate and methionine cycles. This shows the importance of MTHFR within the human body, and identifies the issues that may arise if MTHFR dysfunction occurs[1].
Contents |
Function
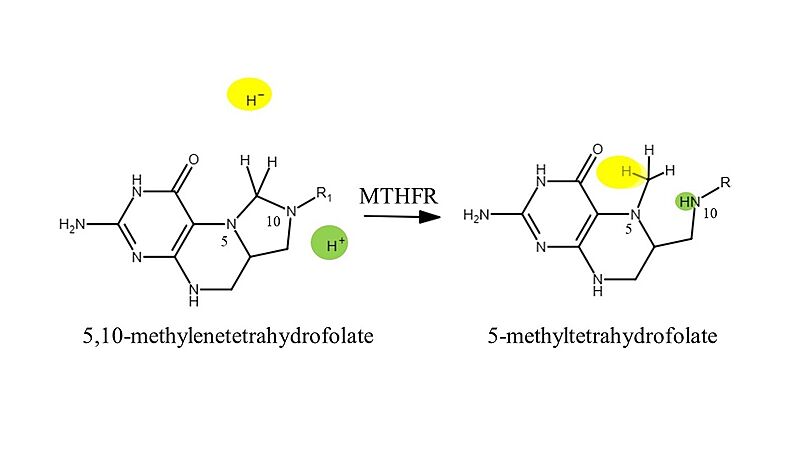
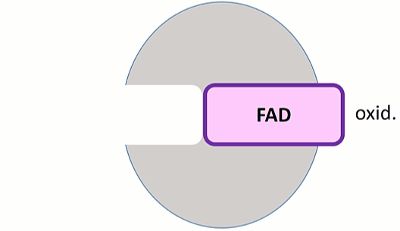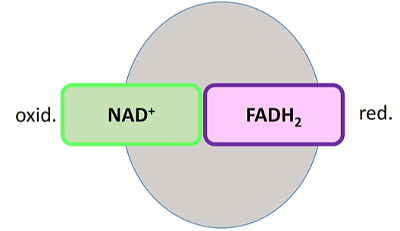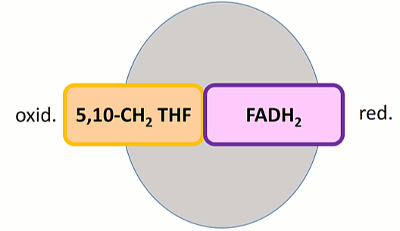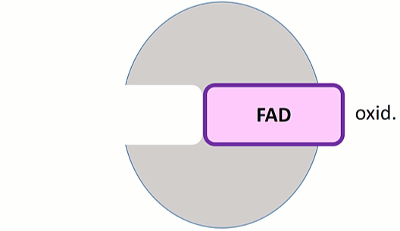
Methylenetetrahydrofolate reductase (MTHFR) enzyme catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate to be recycled back into the folate cycle, and for aiding folate uptake in the body. This reduction reaction requires the cofactor molecule flavin adenine dinucleotide (FAD) and the second substrate nicotinamide adenine dinucleotide phosphate (NADPH) as the electron donor in the reaction. MTHFR has a unique folding structure. Its N-terminal is abundant in serine and acts as a phosphorylation site, its situated in close proximity to it's C-terminal S-adenosyl methionine (SAM) binding site. A linker joins the catalytic domain (N-terminal) to the regulatory domain (C-terminal) for interaction and increases the sensitivity to SAM binding and feedback properties. Additionally, FADH2 is the prosthetic group which acts as an oxidizing or reducing agent depending on the oxidation state of the substrates NADH or 5,10MTHF. When 5,10 MTHF binds FADH2 acts as a reducing agent yielding FAD, and when NADH binds FAD is reduced back to its initial reduced state (FADH2) to allow the cycle to continue.[1]
Mechanism
This reaction mechanism is characterized by a hydride transfer (highlighted in yellow) from the FADH2 to the partially positive 5,10-methyl group. This causes the 5-membered ring to open and nitrogen 10 to receive the electrons that were involved in the C-N10 bond. This allows nitrogen 10 to act as a Brønsted-Lowry base and accept the proton highlighted in green.
5,10-methylenetetrahydrofolate + NADPH + H+ → 5-methyltetrahydrofolate + NADP+
In a second step, FADH2 is regenerated through a hydride transfer from the second substrate, NADPH.
Disease
In addition to the folate cycle, MTHFR is also a major component of the homeostasis of homocysteine in the blood stream. When this homeostasis is disrupted, it results in hyperhomocysteinemia with homocystinuria, or mild hyperhomocysteinemia. Hyperhomocysteinemia is an excess of the amino acid circulating in the body, and is a direct correlation of cardiovascular disease, Alzheimer's disease, depression, and neural tube defects within the fetus. Furthermore, homocystinuria is clinically described as the body's inability to adequately process homocysteine and the amino acid methionine. This dysfunction can be clinically presented with skeletal, vision, and blood clotting abnormalities coupled with learning disorders[2].
Structure
| |||||||||||
3D structures of methylenetetrahydrofolate reductase
Methylenetetrahydrofolate reductase 3D structures
Acknowledgements
Shaylie Albright would like to thank Dr. Karsten Theis for his support and aid in the construction of this page. Thanks to Dr. Kristen Procko for her review and critiques and thanks to Michael O'Shaughnessy, Kia Yang, and Anna Postnikova for their thoughts and contributions on this project.
References
- ↑ 1.0 1.1 Froese DS, Kopec J, Rembeza E, Bezerra GA, Oberholzer AE, Suormala T, Lutz S, Chalk R, Borkowska O, Baumgartner MR, Yue WW. Structural basis for the regulation of human 5,10-methylenetetrahydrofolate reductase by phosphorylation and S-adenosylmethionine inhibition. Nat Commun. 2018 Jun 11;9(1):2261. doi: 10.1038/s41467-018-04735-2. PMID:29891918 doi:http://dx.doi.org/10.1038/s41467-018-04735-2
- ↑ Leclerc D, Sibani S, Rozen R. Molecular biology of methylenetetrahydrofolate reductase (MTHFR) and overview of mutations/polymorphisms. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6561/
- ↑ Pejchal R, Sargeant R, Ludwig ML. Structures of NADH and CH3-H4folate complexes of Escherichia coli methylenetetrahydrofolate reductase reveal a spartan strategy for a ping-pong reaction. Biochemistry. 2005 Aug 30;44(34):11447-57. PMID:16114881 doi:10.1021/bi050533q
- ↑ Lee MN, Takawira D, Nikolova AP, Ballou DP, Furtado VC, Phung NL, Still BR, Thorstad MK, Tanner JJ, Trimmer EE. Functional role for the conformationally mobile phenylalanine 223 in the reaction of methylenetetrahydrofolate reductase from Escherichia coli. Biochemistry. 2009 Aug 18;48(32):7673-85. PMID:19610625 doi:10.1021/bi9007325
Proteopedia Page Contributors and Editors (what is this?)
Shaylie Albright, Karsten Theis, Michal Harel, Michael O'Shaughnessy, Kia Yang, Anna Postnikova