Sandbox1
From Proteopedia
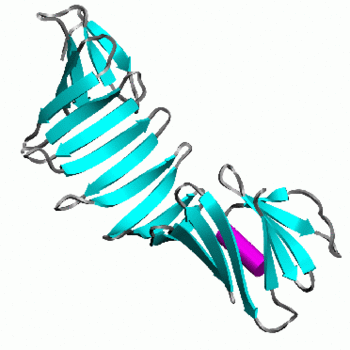
OspA is an outer surface lipoprotein of Borrelia Burgdorferi which is associated with Lyme disease in humans. It is made up of 21 consecutive antiparallel beta-sheets and 1 short alpha helix. [1] OspA plays a great role in causing neural dysfunction of the central nervous system (Lyme neuroborreliosis) and autoimmune Lyme arthritis. Early symptoms of Lyme disease include formation of rash and local infection called erythema migrans.[2] The structure of OspA is widely studied to determine its epitopes and residues, which is essential to develop an effective vaccine against Lyme disease.
Contents |
OspA's Role in Lyme Disease
Lyme disease is the most prevalent human tick-borne disease in North America. Its causative agent, spirochete Borrelia Burgdorferi (B.b.) is transmitted to humans by infected ticks of genus Ixodidae. There are several species of Borrelia Burgdorferi among which B.b. sensu stricto is the only species present in the US that infects 20 to 100 people per 100,1000 people. Other species include B. afzelii, B. garinii, and B. spielmanii that results in about 100 to 130 cases of infections per 100,000 people in Europe and Asia. [2]
Borrelia escapes the host immune system in various ways which includes down regulation of OspA during the feeding process of infected tick on the host. This is important due to OspA’s function as an important adhesive protein in the tic gut. Once inside the host, OspA is up regulated which lead to dysfunction of the central nervous system. [2] Vaccination with OspA signals an immune response that can prevent transmission of B.b. to a mammalian host during the attachment of an infected tick. However, total anti-OspA antibody does not represent the immune protective capacity of sera, but reactivity with a specific epitope of OspA correlates with protective immunity. [3] Other outer surface proteins such as OspB, and OspC are also essential in the successful transmission of B.b. from tick to human host.
Structure of OspA
|
OspA is made up of 273 residues over 21 anti-parallel β-sheets and a single α-helix. It's folded conformation is divided into three main sections: a N-terminus "sandwich," a central region comprising of several β-sheets and a C-terminus "barrel" domain.[3] The folded regions at its ends are connected by a single β-sheet layer in the middle, giving the protein the unique shape of a dumbell.[4] There are at the C-terminus of OspA that are important in binding with the LA-2 Fab antibody, whose interactions provide great insight into vaccine research and effectiveness. These three loops are linearly arranged and form protruding ridge at the C-terminus of OspA. Within these loops, there are where there are distinct variations between the different strains of Borrelia and serve as potential targets for the creation of a broader vaccine.[3]
, (residues 203-220), is important in showing variation amongst the different strains of Borrelia as well as being optimally conformed for binding without steric hindrance. (residues 224-233) and (residues 246-257) are more strongly conserved than Loop 1 but also help to show some variation amongst strains. The LA-2 Fab antibody readily recognizes OspA from B. burgdorferi, but does not recognize that from B. afzelii or B. garinii. Between B. burgdorferi and B. afzelii genetic sequences are generally invariant, but two residues change between the species: in B. burgdorferi is a Glutamine (Gln) in B. afzelii, and in B. burgdorferi is an Alanine (Ala) in B. afzelii. B. garinii has more variation and in addition to the previous two differences, having at least one more difference, where in B. burgdorferi is a Lysine (Lys), and sometimes also has a deletion at B. burgdorferi’s Alanine 208. LA-2 and OspA of B. burgdorferi form a tight interface when binding, and the longer Glutamine (Gln) sidechain found in B. afzelii and B. garinii is more difficult to accommodate, causing less binding. A chimera that was weakly recognized by LA-2 was made with parts of loop 1 from B. burgdorferi, and loops 2 and 3 from B. garinii.[3] Recently, a different kind of chimera has been made which combined the proximal region of B. burgdorferi and distal region of B. afzelii, and was able to successfully protect mice from both species.[5]
Scene List:
OspA's Role in Invasion
Once inside the host, the Borrelia has a great number of mechanisms available to actively suppress the host's immune system response and neutralize its effector mechanisms, such as the expression of another outer surface protein, OspC, which prevents susceptibility to the host's innate immunity and complement systems. Additionally, Borrelia is capable of suppressing many of its surface proteins to reduce its detectability, but can also utilize protective means by temporarily expressing them when needed.
Acute Lyme Neuroborreliosis (LNB)
Acute Lyme Neuroborreliosis (LNB) is part of the second stage of Lyme disease in which the spirochete invades the peripheral and central nervous systems (CNS). Symptoms of LNB include: meningoradiculitis with inflammation of the nerve roots and radiculitis (Bannwarth’s syndrome), lymphocytic meningitis, and cranial and peripheral neuritis. In Europe, the strain predominantly found in the CSF of patients with Bannwarth's syndrome is B. garinii. However, in the United States, Bannwarth's syndrome is rare and the most common manifestations of Lyme neuroborreliosis is meningitis, caused by B. burgdorferi. The presence of OspA in the cerebrospinal fluid (CSF) is responsible for this complex inflammatory response in the brain that leads to the neuroborreliosis.[2]
Evasion and the Extracellular Matrix
The Borrelia are able to hide in the extracellular matrix, allowing it to survive by avoiding leukocytes circulating in the bloodstream. OspA can rapidly bind to plasminogen, which becomes plasmin once activated, and degrades the extracellular matrix. By binding to plasminogen, Borrelia could be exploiting its function and utilizing it to invade the extracellular matrix. However, due to the fact that OspA is downregulated during feeding, and stays unexpressed, a different mechanism may be used instead. Additionally, Borrelia induces the local upregulation of matrix metalloproteinase-9, causing the digestion of the surrounding extracellular matrix. Borrelia can also bind to several proteins in the extracellular matrix, such as fibronectin, integrins or decorin, which can aid in the spread and survival of the spirochetes in these tissues.[2]
Migration Across the Blood-Brain Barrier
It is not fully understood how Borrelia get past the blood-brain barrier, though some researchers suggest a paracellular route, which involves a process using transient tether-type associations, short-term dragging interactions, and stationary adhesion. There is evidence that Borrelia utilizes OspA in the transient tethering stage. The blood-brain barrier is composed of brain microvascular endothelial cells, astrocytes, a basement membrane, pericytes, and neurons. OspA is a major adherent molecule to brain microvascular cells by binding to the CD40 receptors outside, which results in events that are typically seen when leukocytes cross the blood brain barrier. Activation of CD40 receptors leads to the production of proinflammatory cytokines and enhanced expression of ICAM-1, E-selectin and VCAM-1, resulting in increased cell binding, and the formation of fenestrations due to increased vascular endothelial growth factor, and vascular permeability factor. OspA might be mimicking leukocytes in order to cross the blood-brain barrier. However not all strains of Borrelia can utilize OspA to do this, OspA only contributes about 70% to adherence, and other Borrelia proteins are also needed in this process. It has also been seen that OspA mediates the adhesion of Borrelia to murine neural and glial cell lines. [6]
Role in Inflammation
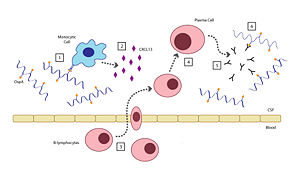
There are six steps involved in the host's inflammatory response to OspA: [2]
- When the Borrelia enter the host’s CNS they encounter several different types of immune cells such as monocytes, macrophages, and dendritic cells. While in the CSF, outer surface protein A (OspA) is upregulated and it’s increased expression promotes recognition by a specific receptor on a monocyte.
- The OspA-bound monocyte then releases proinflammatory cytokines (i.e. interferon), as well as chemokines, such as CXCL13. In patients with LNB, there is an observed increase in the levels of these cytokines and chemokines in their CSF. The production of chemokines leads to the recruitment of other immune cells to the site of infection.
- B-lymphocytes respond to the new concentration gradient of CXCL13 between the blood and CSF and migrate into the CSF.
- B-lymphocytes undergo receptor-mediated endocytosis, consuming the OspA antigens present in the CSF, thereby triggering its activation. The B-lymphocytes then are able to differentiate and mature into plasma cells.
- The plasma cells create large quantities of anti-OspA antibodies specific to this strain of Borrelia and release them into the CSF.
- The anti-OspA antibodies will then bind to the OspA on the spirochete’s membrane, thus killing the Borrelia.
This process is two-sided in the sense that the OspA aids in the pathogenesis of new symptoms (neuroborreliosis) through the chemokine’s actions, as well as initiating the signaling cascade to destroy itself.
OspA Vaccination
|
Risk of developing Lyme disease can be mitigated by staying clear of areas with populations of ticks, wearing proper attire to minimize easily bitten areas of the body, and using insect repellents containing DEET (N,N-diethy-m-toluamide). However, another effective means for prevention could be possible by using an outer surface protein from Borrelia in the creation of a vaccine.[7]
The membrane composition of Borrelia is abundant in both OspA and OspB, and the two proteins share a 53% similarity in their primary sequences. Both OspA and OspB are expressed in the tick's gut and downregulated during feeding and aid in its survivability; however, OspA is overall less varied and reactive than OspB, which has greater variability.[8] The relatively conserved sequence of OspA thus lends itself better to study and application toward the development of a vaccine for a broader range of Borrelia strains in the treatment of Lyme disease than that of OspB. The first vaccine used a purified recombinant form of OspA and functioned in blocking transmission of the spirochetes expressing OspA from tick to host during feeding, killing them while still attached to the tick's gut.[9][10] The vaccine, Lymerix, had shown 76% and 92% effectiveness in separate clinical trials in which patients were treated for two years following a three-dose schedule. However, the vaccination was suspended from use in 2002 when opponents claimed the IgG antibodies for OspA were associated with the onset of severe chronic arthritis, as well as other side effects affecting immunity.[9][11] This fact, in conjunction with the desire for a more widespread vaccine treating multiple strains of Borrelia, has spurred research towards a new vaccine.
To address the concerns of vaccine with broader protection, creation of a chimera, mixing the OspA of different strains of Borrelia would be ideal. Study of the epitope of and its with the murine monoclonal antibody have proved useful in determining effectiveness of a given vaccine trial as high levels of antibodies in test sera compete against LA-2 for binding with OspA. LA-2 makes direct contact with three exposed loops of the C-terminus of OspA. The recognition of OspA by LA-2 requires an induced fit mechanism where these three loops undergo conformational changes to optimize their interaction in the complex. [3]
Scene List for the OspA:LA-2 Complex
- Fab antibody (Bluish regions indicate heavy chains (chains B & D of 1FJ1) and greenish regions indicate light chains (chains A & C of 1FJ1))
- proteins in complex with the LA-2 Fab antibody (chains E & F of 1FJ1)
- of the OspA antigen : LA-2 Fab antibody interactions
References
- ↑ Luft BJ, Dunn JJ, Lawson CL. Approaches toward the directed design of a vaccine against Borrelia burgdorferi. J Infect Dis. 2002 Feb 15;185 Suppl 1:S46-51. PMID:11865439 doi:10.1086/338463
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The pathogenesis of lyme neuroborreliosis: from infection to inflammation. Mol Med. 2008 Mar-Apr;14(3-4):205-12. PMID:18097481 doi:10.2119/2007-00091.Rupprecht
- ↑ 3.0 3.1 3.2 3.3 3.4 Ding W, Huang X, Yang X, Dunn JJ, Luft BJ, Koide S, Lawson CL. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol. 2000 Oct 6;302(5):1153-64. PMID:11183781 doi:10.1006/jmbi.2000.4119
- ↑ Makabe K, Tereshko V, Gawlak G, Yan S, Koide S. Atomic-resolution crystal structure of Borrelia burgdorferi outer surface protein A via surface engineering. Protein Sci. 2006 Aug;15(8):1907-14. Epub 2006 Jul 5. PMID:16823038 doi:10.1110/ps.062246706
- ↑ Livey I, O'Rourke M, Traweger A, Savidis-Dacho H, Crowe BA, Barrett PN, Yang X, Dunn JJ, Luft BJ. A new approach to a Lyme disease vaccine. Clin Infect Dis. 2011 Feb;52 Suppl 3:s266-70. PMID:21217174 doi:10.1093/cid/ciq118
- ↑ Pulzova L, Kovac A, Mucha R, Mlynarcik P, Bencurova E, Madar M, Novak M, Bhide M. OspA-CD40 dyad: ligand-receptor interaction in the translocation of neuroinvasive Borrelia across the blood-brain barrier. Sci Rep. 2011;1:86. Epub 2011 Sep 8. PMID:22355605 doi:10.1038/srep00086
- ↑ Nigrovic LE, Thompson KM. The Lyme vaccine: a cautionary tale. Epidemiol Infect. 2007 Jan;135(1):1-8. Epub 2006 Aug 8. PMID:16893489 doi:10.1017/S0950268806007096
- ↑ Becker M, Bunikis J, Lade BD, Dunn JJ, Barbour AG, Lawson CL. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J Biol Chem. 2005 Apr 29;280(17):17363-70. Epub 2005 Feb 15. PMID:15713683 doi:10.1074/jbc.M412842200
- ↑ 9.0 9.1 Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005 May;3(5):411-20. PMID:15864264 doi:10.1038/nrmicro1149
- ↑ Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008 Nov;76(11):5228-37. Epub 2008 Sep 8. PMID:18779341 doi:10.1128/IAI.00410-08
- ↑ Plotkin SA. Correcting a public health fiasco: The need for a new vaccine against Lyme disease. Clin Infect Dis. 2011 Feb;52 Suppl 3:s271-5. PMID:21217175 doi:10.1093/cid/ciq119
External Links
- World Health Organization: Lyme Disease
- PubMed Health: Lyme Disease
- American Lyme Disease Foundation